Session Information
Date: Friday, November 6, 2020
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The efficacy of the interleukin (IL)-23 subunit p19 inhibitor guselkumab (GUS) for psoriatic arthritis (PsA) has recently been demonstrated in two Phase 3 trials (DISCOVER‑1 & -2) but has not been evaluated versus existing targeted therapies for PsA. The objective of this study was to compare GUS to targeted therapies for PsA through network meta-analysis (NMA).
Methods: A systematic literature review was performed to identify PsA randomized controlled trials from 2000 to 2018. Bayesian NMAs were performed to compare treatments on American College of Rheumatology (ACR) 20/50/70 response, Psoriasis Area Severity Index (PASI) 75/90/100 response, Health Assessment Questionnaire Disability Index (HAQ-DI) score, modified van der Heijde-Sharp (vdH-S) score, adverse events (AEs) and serious adverse events (SAEs). For ACR 20 and PASI 75 response outcomes, analyses stratified according to previous exposure to biologics. Analyses adjusted for placebo response via meta-regression on baseline risk when feasible. Results are summarized by ranking treatments according responses derived from NMAs, with conclusions (ie, comparable or better/worse) derived from overlap of pairwise 95% credible intervals (CrI) between treatments.
Results: Twenty-four Phase 3 studies were included in NMAs. Studies were placebo-controlled up to 24 weeks and evaluated 13 targeted therapies for PsA. Forest plot of relative risks (RR) versus placebo for ACR 20 and PASI 75 according to previous exposure to biologics are presented in Figure 1 and Figure 2, respectively. Forest plot of RRs versus placebo for AEs are presented in Figure 3. For ACR 20 response in the bio-naïve population, GUS 100 mg every 4 weeks (Q4W) and every 8 weeks (Q8W) ranked 5th and 8th out of 19 interventions and were comparable to IL-17 inhibitor (IL-17i) and subcutaneous tumor necrosis factor inhibitor (TNFi) agents. For ACR 20 response in the bio-experienced population, GUS Q4W and Q8W ranked 2nd and 3rd out of 14 interventions and were considered comparable to other active agents. Similar results were seen in mixed analyses of ACR 50 & 70. For PASI 75 response in the bio-naïve population, GUS Q4W and Q8W ranked 1st and 2nd out of 18 interventions and were better than most other agents. For PASI 75 in the bio-experienced population, GUS Q4W and Q8W ranked 3rd and 2nd out of 11 interventions and were better than several other agents. Similar results were seen in mixed analyses of PASI 90 & 100. For HAQ‑DI score, GUS Q4W and Q8W ranked 6th and 11th out of 20 interventions and were comparable to IL‑17i and subcutaneous TNFi agents. For vdH-S score, GUS Q4W and Q8W ranked 3rd and 10th out of 18 interventions, with Q8W comparable to most IL-17i and TNFi agents and Q4W likely to provide a benefit over IL-17i agents and most TNFi agents. For both AEs & SAEs, GUS Q4W and Q8W were comparable to most other agents.
Conclusion: GUS provides joint arthritis efficacy (ACR responses and modified vdH-S score), physical function (HAQ-DI score), and safety outcomes that is comparable to most targeted PsA treatments. For PASI outcomes, GUS is considered better than most other targeted PsA treatments.
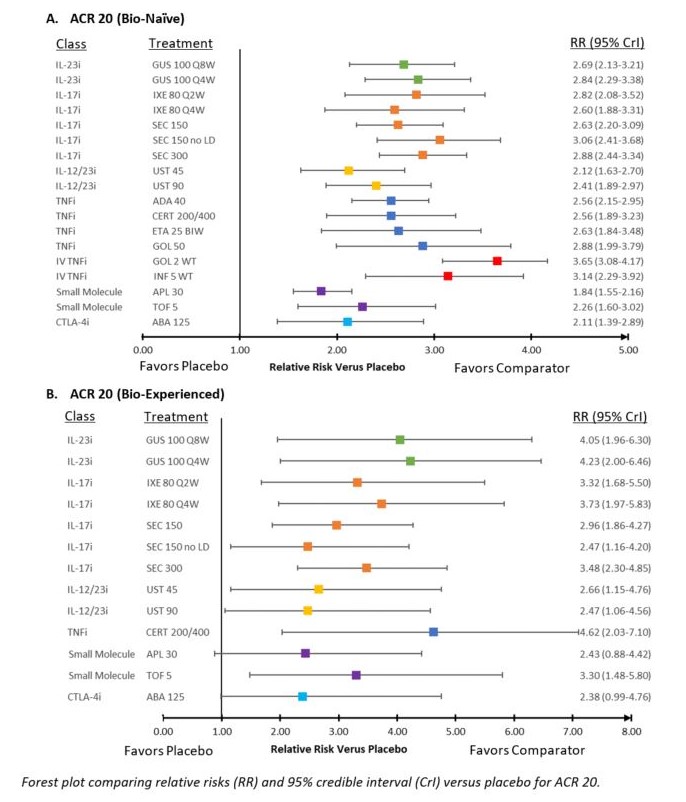 Figure 1: Forest Plot of ACR 20 Response Versus Placebo In Bio-Naïve and Bio-Experienced Populations
Figure 1: Forest Plot of ACR 20 Response Versus Placebo In Bio-Naïve and Bio-Experienced Populations
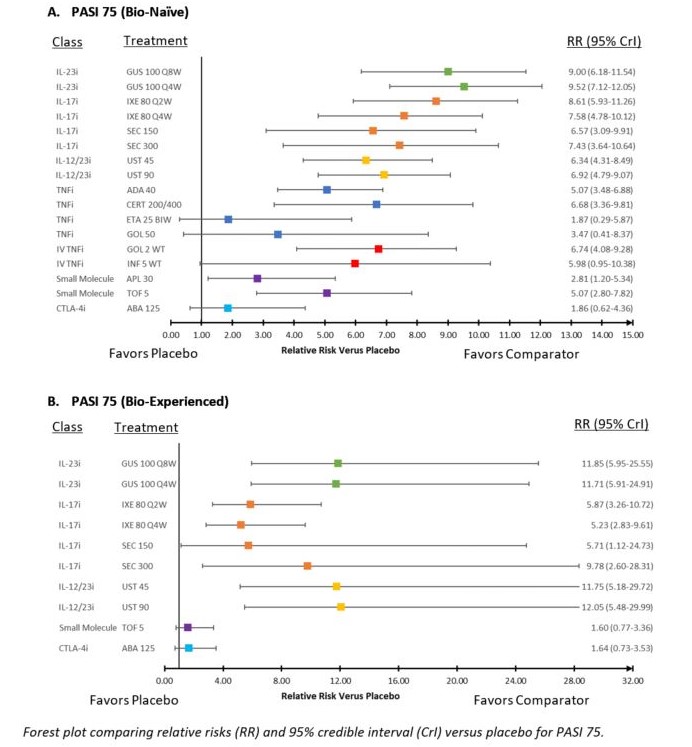 Figure 2: Forest Plot of PASI 75 Response Versus Placebo In Bio-Naïve and Bio-Experienced Populations
Figure 2: Forest Plot of PASI 75 Response Versus Placebo In Bio-Naïve and Bio-Experienced Populations
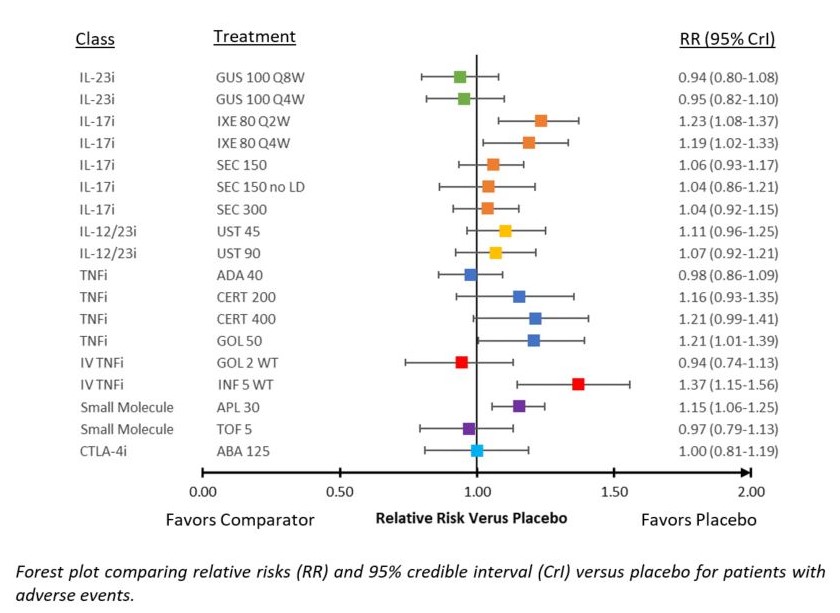 Figure 3: Forest Plot of Adverse Events Versus Placebo
Figure 3: Forest Plot of Adverse Events Versus Placebo
To cite this abstract in AMA style:
Mease P, McInnes I, Eaton K, Peterson S, Disher T, Chakravarty S, Karyekar C, Nair S, Boehncke W, Ritchlin C. Comparative Efficacy of Guselkumab in Patients with Psoriatic Arthritis: Results from Systematic Literature Review and Network Meta-Analysis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/comparative-efficacy-of-guselkumab-in-patients-with-psoriatic-arthritis-results-from-systematic-literature-review-and-network-meta-analysis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-efficacy-of-guselkumab-in-patients-with-psoriatic-arthritis-results-from-systematic-literature-review-and-network-meta-analysis/
