Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Among the treatment options for PsA, IL-12/23 inhibition with ustekinumab (UST) was the first new biologic mode of action after TNF inhibitors (TNFi). Few data are available from real-world settings comparing the effectiveness of UST to standard TNFi. The objective of this study was to investigate 6-mo treatment responses to UST or TNFi in the 8-country, real-world PsABio study, especially achievement of LDA and remission.
Methods: The PsABio study (NCT02627768) evaluates effectiveness, tolerability and persistence of 1st, 2nd or 3rd-line UST or TNFi in PsA. Proportions of patients (pts) reaching MDA/VLDA and cDAPSA were evaluated. Baseline (BL) and 6-mo data were compared among pts receiving UST or TNFi for full 6 mo (completers), and ITT analysis including pts switching/stopping original treatment during 6-mo observation period, imputed as non-responders. Propensity score (PS) was used to adjust for BL covariates in country, age, gender, BMI, smoking (yes/no), co-morbidities (cardiovascular/metabolic syndrome), PsA type (axial, polyarticular, oligoarticular), psoriasis BSA, disease duration, cDAPSA, PsAID-12, dactylitis, enthesitis, FiRST score, line of biologic (b)DMARD, synthetic DMARD use, and steroid or NSAID use. Factors potentially modifying PS-adjusted treatment effect were investigated.
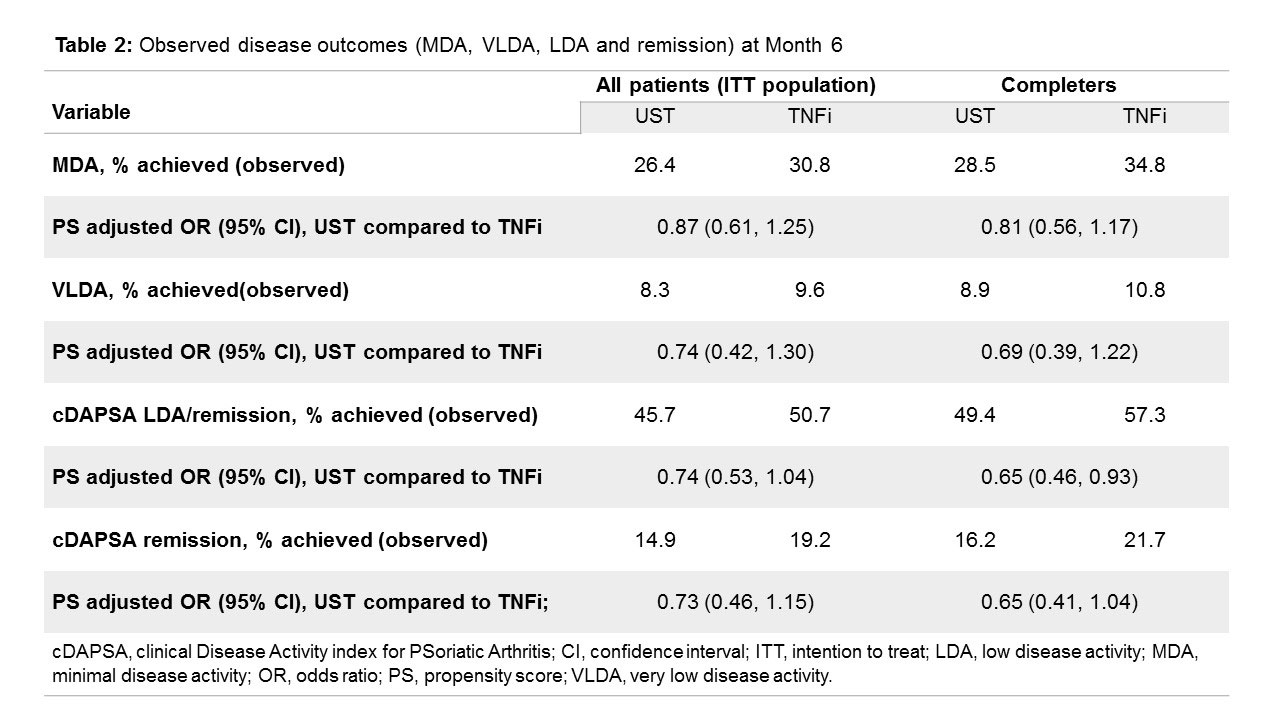
Results: For N=930 pts, 796 (93.0% and 93.6% of UST- and TNFi-treated, respectively) had evaluable data at BL and 6 mo (Table 1). ITT analysis (n=868) included pts who had stopped/switched before 6 mo (UST: n=28/426 [6.6%], TNFi: n=44/442 [10.0%]). For MDA assessment, 761 (ITT) and 701 (completers) had data for both visits. Both UST and TNFi led to achievement of MDA (up to 35%), VLDA (up to 11%), cDAPSA LDA (up to 57%) and cDAPSA remission (up to 22%) (Table 2). At BL, significant differences between UST and TNFi groups existed in age (higher on UST), line of treatment (UST more frequently 3rd-line), NSAID use (lower in UST), methotrexate use (less concomitant treatment in UST), FiRST score (more chronic widespread pain in UST), and skin involvement (higher in UST). After PS adjustment for BL differences, odds ratios (OR) for reaching MDA, VLDA, cDAPSA LDA or remission at 6 mo, were not statistically different between UST and TNFi (Table 2). In the ITT set, pts with low-grade skin disease (BSA< 3%) and those with oligoarticular disease had a lower chance of achieving MDA when given UST (OR: 0.45 [0.29, 0.85] and 0.62 [0.39, 0.97] respectively) compared with TNFi, but responses to UST tended to be better in pts with higher grade skin disease ( >10% of BSA) and with polyarticular disease at BL (OR: 2.08 [0.88, 4.95] and 1.57 [0.88, 2.82] respectively). Similar results were seen in the analysis obtained with cDAPSA LDA or remission as treatment target.
Conclusion: UST and TNFi, when used as 1st-, 2nd- or 3rd-line bDMARD in a routine care setting, provide clinically relevant regression of disease signs and symptoms and allow achievement of MDA, LDA or remission in many PsA pts after 6 mo of treatment. After PS adjustment for BL imbalances, clinical results achieved were comparable between UST and TNFi.
To cite this abstract in AMA style:
Smolen J, Athanassiou P, Bergmans P, Bondareva I, De Vlam K, Gremese E, Joven-Ibáñez B, Korotaeva T, Noël W, Nurmohamed M, Sfikakis P, Siebert S, Smirnov P, Theander E, Gossec L. Comparative Effectiveness of Ustekinumab and TNF Inhibitors in Patients with Psoriatic Arthritis in a Real-world, Multicenter Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/comparative-effectiveness-of-ustekinumab-and-tnf-inhibitors-in-patients-with-psoriatic-arthritis-in-a-real-world-multicenter-study/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-effectiveness-of-ustekinumab-and-tnf-inhibitors-in-patients-with-psoriatic-arthritis-in-a-real-world-multicenter-study/