Session Information
Date: Friday, November 6, 2020
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The treatment of psoriatic arthritis has evolved with the addition of agents targeting a different cytokine, Il-17 introduced in 2016. It has proven to be more effective than anti-TNF in controlling the cutaneous manifestation of psoriasis. Recent data have not shown clear superiority on the articular manifestations [EXCEED Study].
Methods: Patients diagnosed with PsA enrolled in RHUMADATA® were selected if they gave informed consent and were treated with secukinumab (SEC), adalimumab (ADA) or other TNF inhibitors (TNFi) on or after January 1, 2016. RHUMADATA® provides socio-demographics, comorbidities, patient-reported outcomes, disease activity indices, laboratory variables, rheumatologist exam report data, use of concomitant medications and PsA treatment. Kaplan-Meier survival curves and proportional hazard models were used to analyze time to treatment cessation.
Results: A total of 305 patients with PsA were extracted from RHUMADATA®. One hundred and eighteen were treated with SEC, 75 with ADA and 112 with other TNFi (certolizumab, golimumab or infliximab. etanercept was not included as it is not an atibody targetting TNFi). Overall, 54.1% were women, average age at diagnosis and disease duration at treatment initiation were respectively 44.3 (±standard deviation (STD)=12.4) and 6.3 (7.9) years. The age-adjusted Charlson Comorbidity Index (aCCI) was 1.4 (1.8). The proportion of patients diagnosed or treated for diabetes and hypertension were 13.4% and 37.7%. The patient’s assessment of disease activity, pain and fatigue were 5.5 (2.7), 6.0 (2.8) and 5.5 (3.1). Overall, BASDAI and BASFI scores were 5.6 (2.5) and 4.4 (2.8). The mean number of affected fingers and toes (dactylitis) and enthesitis were 0.9 (1.5) and 0.9 (1.7), respectively. SEC, ADA and TNFi were used in first intention in 25.4%, 61.3% and 37.5% of patients, respectively. CDAI at treatment initiation was 18.5 (11.0), 21.0 (10.2) and 16.6 (8.8) in the SEC, ADA and TNFi groups, respectively. At one-year CDAI, values were 6.1 (7.5), 5.0 (5.4) and 4.9 (3.1). Kaplan-Meier log-rank p-values comparing the three groups in all (Figure 1), first and second, and third+ intentions were 0.5701, 0.6631 and 0.3246, respectively. No pairwise comparisons of treatment (SEC vs. ADA, SEC vs. TNFi, ADA vs. TNFi) showed better retention for any agent. Pairwise comparison of these agents in monotherapy numerically favored SEC over ADA (Figure 2) but failed to reach statistical significance (log-rank p-value=0.5203), and SEC over TNFi (Figures 3) statistically favored SEC over ADA (log-rank p-value=0.0150). A Cox proportional hazard model adjusting for age at diagnosis, disease duration, aCCI, the number of prior advanced therapies, and concomitant use of MTX at treatment initiation as well as selected treatment demonstrated a statistically better retention among patients treated with MTX (Adjusted hazard ratio and 95% confidence interval: 0.487 [0.347, 0.683]. Adjusted treatment-MTX interaction terms were non-significant.
Conclusion: Secukinumab in observational data have shown similar efficacy over ADA and other TNFi. The use of these treatments in monotherapy showed similar retention for SEC over ADA , and impreoved retention for SEC over TNFi.
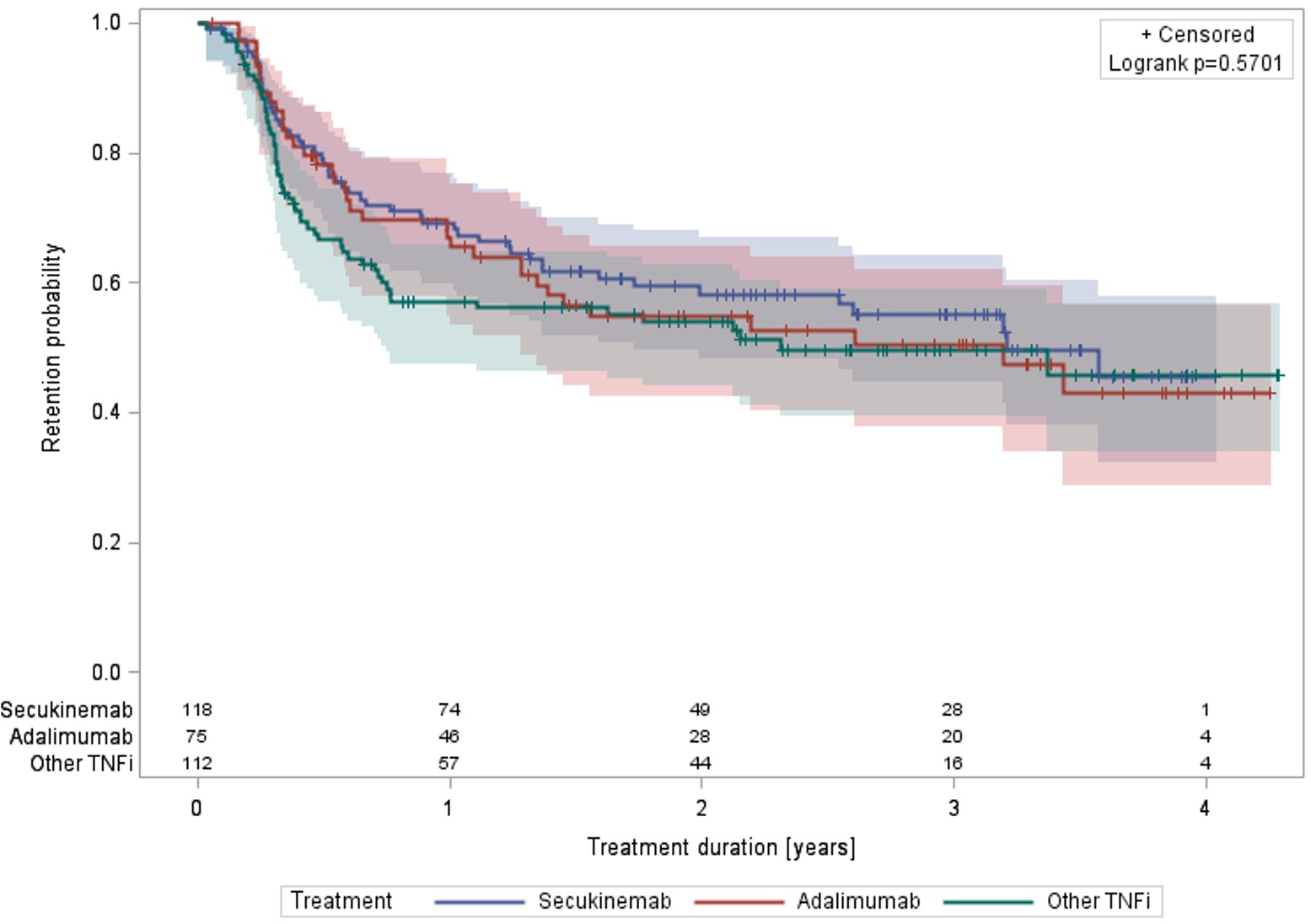 Retention of SEC, ADA and TNFi (all intentions).
Retention of SEC, ADA and TNFi (all intentions).
 Retention of SEC and ADA in monotherapy (all intentions).
Retention of SEC and ADA in monotherapy (all intentions).
 Retention of SEC and TNFi in monotherapy (all intentions).
Retention of SEC and TNFi in monotherapy (all intentions).
To cite this abstract in AMA style:
Choquette D, Choquette Sauvageau L, Bessette L, Ferdinand I, Haraoui B, Massicotte F, Pelletier J, Raynauld J, Rémillard M, Sauvageau D, Villeneuve �, Coupal L. Comparative Effectiveness of Secukinumab, Adalimumab and Other Tumor Necrosis Factor Inhibitors Used with or Without Methotrexate in the Treatment of Psoriatic Arthritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/comparative-effectiveness-of-secukinumab-adalimumab-and-other-tumor-necrosis-factor-inhibitors-used-with-or-without-methotrexate-in-the-treatment-of-psoriatic-arthritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-effectiveness-of-secukinumab-adalimumab-and-other-tumor-necrosis-factor-inhibitors-used-with-or-without-methotrexate-in-the-treatment-of-psoriatic-arthritis/
