Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: In patients (pts) who have failed 1 or more TNF-α inhibitors (TNFi), there is little data to guide clinical decision making in terms of changing mechanism of action or prescribing another TNFi. The objective of this study was to examine the comparative effectiveness of rituximab (RTX) versus a subsequent TNFi in RA pts with prior TNFi exposure using data from the Consortium of Rheumatology Researchers of North America (CORRONA), a multi-center observational registry within the United States.
Methods: Using CORRONA data spanning from 10/8/2001 through 10/31/2012, we identified RTX-naïve RA pts with prior TNFi use who were initiating RTX or a subsequent TNFi, had a 1-year follow-up visit and had Clinical Disease Activity Index (CDAI) measurements at baseline and 1-year follow-up. A propensity score (PS) for TNFi vs. RTX was estimated using baseline clinical characteristics. The PS distributions were trimmed to exclude pts who fell outside the region of common support (n = 2). Effectiveness outcomes at 1 year included achievement of low disease activity (LDA, CDAI ≤ 10), achievement of modified ACR (mACR) 20/50/70 and decrease in mHAQ of 0.25. Nonresponse was imputed for pts who switched biologics. A sensitivity analysis using stratified matching by 1 vs. ≥ 2 prior TNFi and matching by PS was performed. Multivariable regression models adjusted for baseline parameters with a standardized difference > 0.1, baseline CDAI, steroid use, number of prior TNFi (1 vs. ≥ 2), and concomitant MTX use.
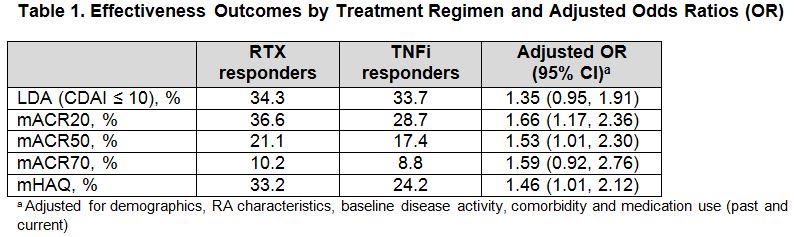
Results: 265 RTX pts and 737 TNFi pts met the inclusion criteria for the analysis. At baseline, both groups were mostly female (79–81%) with a mean age of 56-58 years, mean CDAI of 25.2-27.3 and mean mHAQ of 0.64-0.78. RTX pts had longer duration of disease (15 vs 11 years), greater prior use of ≥ 2 TNFi’s (63% vs 27%) and greater prior use of non-TNF biologics (41% vs 13%) compared with TNFi pts. Results of comparative effectiveness analyses are presented in Table 1. In the PS-trimmed population, RTX treatment was estimated to be associated with a 35-66% increased likelihood of response compared with TNFi treatment, however this was only significant for mACR20, mACR50 and mHAQ. In the TNFi-stratified matched and PS-matched population, RTX treatment was associated with a significantly increased likelihood of achieving LDA compared with TNFi treatment (OR 1.54 [95% CI: 1.01 to 2.35]). During the 1-year follow-up, 16.2% of RTX pts and 29.4% of TNFi pts switched biologics.
Conclusion: In a population of RA pts with prior exposure to TNFi, RTX initiators had longer duration of active disease and had more prior biologic use compared with TNFi initiators. Across measures of disease activity and function, there was an estimated 35-66% increased likelihood of response in pts treated with RTX compared with those treated with TNFi. Fewer RTX pts than TNFi pts were switched to another biologic over the 1-year follow-up period.
Disclosure:
L. R. Harrold,
CORRONA ,
2;
G. W. Reed,
CORRONA ,
3;
R. P. Magner,
University of Massachusetts Medical School ,
3;
A. Shewade,
Genentech, Inc.,
3;
A. John,
Genentech, Inc.,
3;
J. D. Greenberg,
CORRONA ,
1,
CORRONA, AstraZeneca, Novartis, Pfizer,
5;
J. M. Kremer,
CORRONA ,
1,
CORRONA ,
3.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-effectiveness-of-rituximab-vs-subsequent-anti-tumor-necrosis-factor-in-patients-with-rheumatoid-arthritis-with-prior-exposure-to-tnfi/