Session Information
Date: Tuesday, November 14, 2023
Title: Abstracts: Osteoporosis & Metabolic Bone Disease – Basic & Clinical Science
Session Type: Abstract Session
Session Time: 4:00PM-5:30PM
Background/Purpose: Although clinical trials have shown that denosumab (Dmab) significantly increases bone mineral density at key skeletal sites more than zoledronic acid (ZA), evidence from randomized trials evaluating fracture outcomes is lacking. This retrospective cohort study evaluated the comparative effectiveness of Dmab versus ZA in reducing fracture risk among women with postmenopausal osteoporosis (PMO) in the U.S.
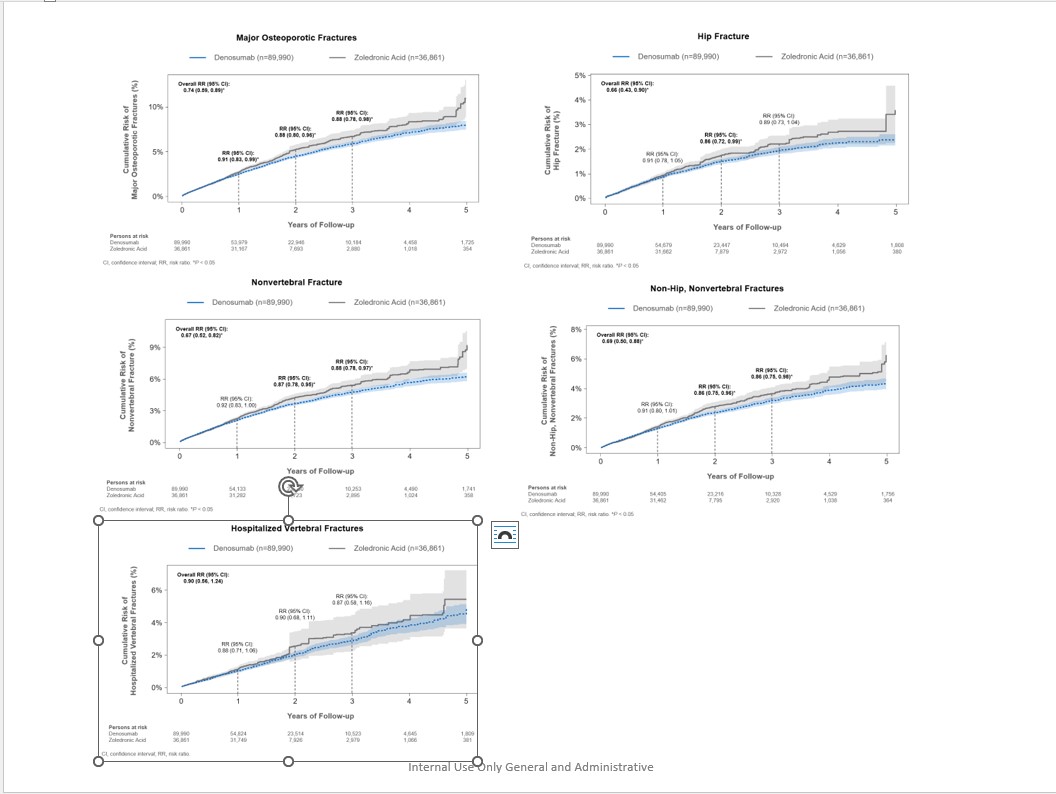
Methods: Female Medicare fee-for-service beneficiaries ≥ 66 years of age who newly initiated Dmab (n=89,990) or ZA (n=36,861) between Jan 1, 2012 to Dec 31, 2018 with no prior history of osteoporosis treatment were followed from treatment initiation (index date) until the first instance of a given fracture outcome, treatment discontinuation (defined as the end of exposure according to usual dosing intervals + 60-day gap) or switch, Medicare disenrollment, death, end of available data (Dec 31, 2019), or 5 years post-index date. A doubly robust inverse-probability of treatment (weights estimated from multivariate logistic regression models) and censoring (weights estimated from multivariate Cox Proportional Hazards regression models) weighted function was used to estimate the relative risk (RR) associated with the use of Dmab compared with ZA for major osteoporotic (MOP; nonvertebral and hospitalized vertebral), hip, nonvertebral (NV; includes hip, humerus, pelvis, radius/ulna, other femur), non-hip, nonvertebral (NHNV), and hospitalized vertebral (HV) fractures for the overall study period and by year of follow-up.
Results: Over a maximum of 5 years of follow-up, Dmab reduced the risk of MOP by 26% (RR=0.74; 95% CI: 0.59-0.89), hip by 34% (0.66; 0.43-0.90), NV by 33% (0.67; 0.52-0.82), and NHNV by 31% (0.69; 0.50-0.88), and HV fractures by 10% (0.90; 0.56-1.24) compared with ZA (Figure). Over time, Dmab reduced the risk of MOP fractures by 9% (0.91; 0.83-0.99) at year 1, 12% (0.88; 0.80-0.96) at year 2, and 12% (0.88; 0.78-0.98) at year 3. An increase in the magnitude of fracture risk reduction with increasing duration of exposure was also observed for other NV outcomes.
Conclusion: In a cohort of over 125,000 treatment-naive women with PMO, we observed robust, clinically meaningful reductions in the risk of MOP, hip, NV, and NHNV fractures for patients on Dmab compared to ZA, with greater reductions in fracture risk with longer duration of exposure.
To cite this abstract in AMA style:
Curtis J, Arora T, Liu Y, Brunetti V, Lin T, Spangler L, Stad R, McDermott M, Bradbury B, Kim M. Comparative Effectiveness of Denosumab versus Zoledronic Acid Among Postmenopausal Women with Osteoporosis in the U.S. Medicare Program [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/comparative-effectiveness-of-denosumab-versus-zoledronic-acid-among-postmenopausal-women-with-osteoporosis-in-the-u-s-medicare-program/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-effectiveness-of-denosumab-versus-zoledronic-acid-among-postmenopausal-women-with-osteoporosis-in-the-u-s-medicare-program/