Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose:
With the availability
of multiple biologic agents, each with different modes of action, use of real
world registries provide the manner in which to examine comparative
effectiveness in the absence of head-to-head clinical trials inform physicians
how they might be used for the treatment of rheumatoid arthritis
Methods: Arthritis Registry Monitoring Database
(ARMD) has been collecting prospective patient data since 2005 in all patients
seen in routine care. Each patient in this setting (with any diagnosis)
completes a 2-sided, 1-page MDHAQ (multidimensional health assessment
questionnaire) at every visit while waiting to see the physician in the
infrastructure of clinical care. The MDHAQ includes scales for physical
function, pain, patient global estimate (PATGL), fatigue, and a self-report
RADAI painful joint count. Usage of the biologic medications abatacept,
adalimumab, certolizumab, etanercept, infliximab, rituximab and tocilizumab
along with self-reported disease activity and clinic measures were abstracted. Treatments
were considered to be independent of each other as no individual received
biologic medications in combination. Time to first response defined as an
improvement in RAPID3 of at least 3.6 was calculated; change from biologic
medication initiation to first response for self-reported disease activity and
clinic measures was estimated. For those individuals with no response, time to
last follow-up was calculated and this was defined as lack of efficacy.
Differences in time to first response between biologic medications versus lack
of efficacy were estimated using competing risks proportional hazards model,
adjusting for age, duration of disease, baseline RAPID3.
Results:
4217
encounters were reviewed for this analysis. A total of 1789 treatment observations
were abstracted in 316 subjects. For those subjects with a baseline RAPID3 ≥
3.6, average age of the cohort was 51.5 years (±14.5), average duration 9.3
years (±9.3), 213 (85%) were female, and average baseline RAPID3 was 14.8 (±6.3).
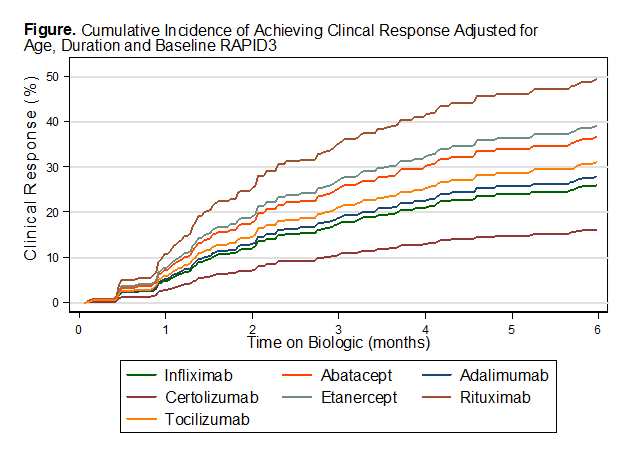
Using infliximab as a reference, improved efficacy was estimated with abatacept
(SHR = 1.5), etanercept (SHR = 1.6) and rituximab (SHR=2.3) adjusting for age,
duration and baseline RAPID3. Increased age led to a decreased likelihood of
response (SHR=0.99, P=0.045). As expected, the higher the baseline RAPID3, the likelihood
of a positive treatment response also increased (SHR=1.09, P<0.001). Adalimumab
and tocilizumab had some improved efficacy, while certolizumab had decreased
efficacy compared to infliximab.
Conclusion:
Our
data suggest that there are no major differences in efficacy of adalimumab,
abatacept, etanercept and infliximab and time to response when treating RA
patients. With no difference in clinical outcomes, most treatment decisions may
be based on ease of use and safety data of respective biologics agents when
they are being considered for RA treatment.
To cite this abstract in AMA style:
Yazici Y, Bernstein H, Swearingen C. Comparative Effectiveness and Time to Response Among Abatacept, Adalimumab, Certolizumab, Etanercept, Infliximab, Rituximab and Tocilizumab in a Real World Routine Care Registry [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/comparative-effectiveness-and-time-to-response-among-abatacept-adalimumab-certolizumab-etanercept-infliximab-rituximab-and-tocilizumab-in-a-real-world-routine-care-registry/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparative-effectiveness-and-time-to-response-among-abatacept-adalimumab-certolizumab-etanercept-infliximab-rituximab-and-tocilizumab-in-a-real-world-routine-care-registry/