Session Information
Date: Monday, November 8, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster II: Psoriatic Arthritis I (1329–1363)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
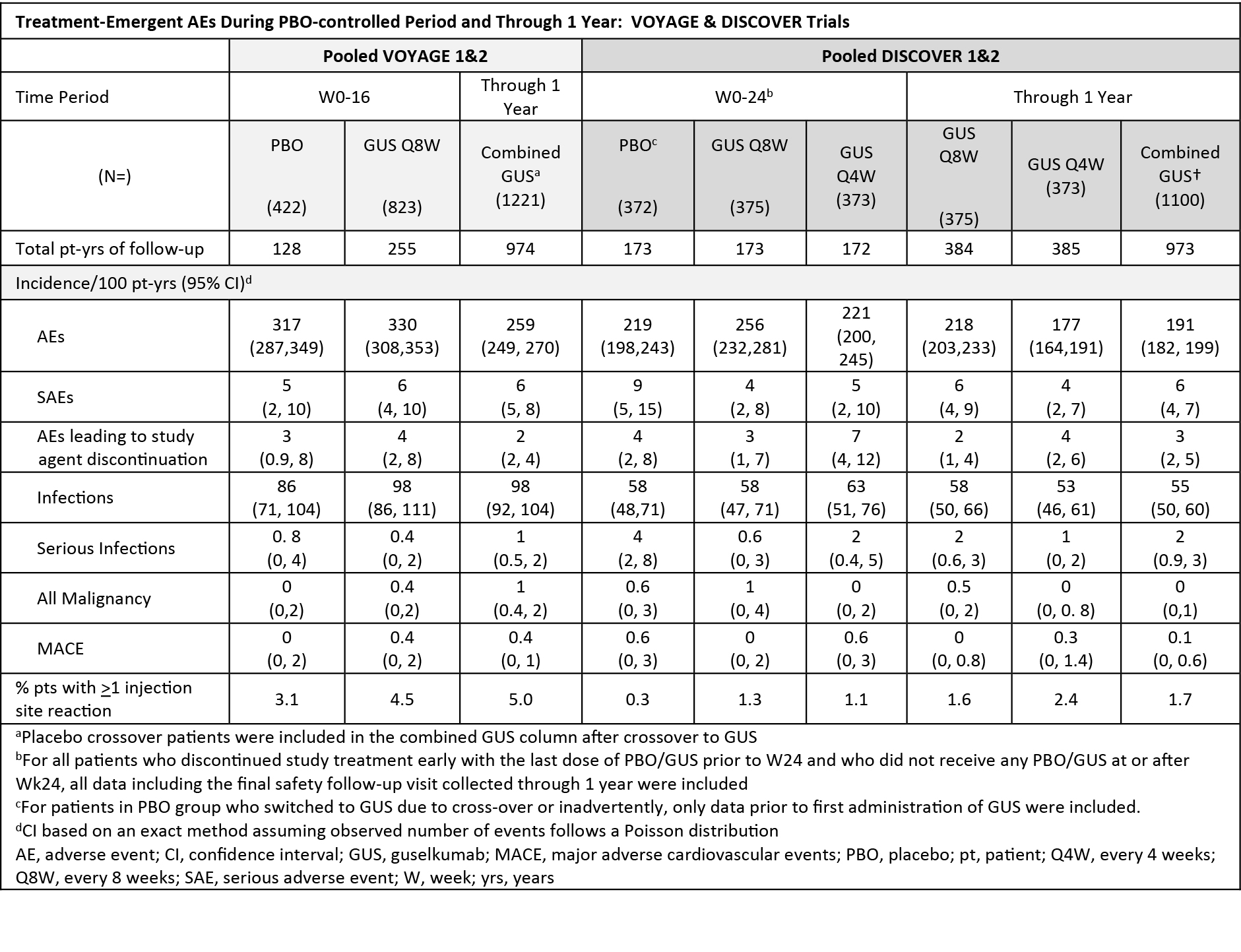
Background/Purpose: DISCOVER 1&2 (PsA) and VOYAGE 1&2 (psoriasis [PsO]) are Phase 3 trials of guselkumab (GUS). Here we compared safety results through up to 1 year of GUS in PsA and PsO patients.
Methods: In DISCOVER 1&2, 1120 patients with active PsA despite standard therapy were treated. Most patients were biologic-naïve; ~30% in DISCOVER 1 had previous exposure to 1-2 TNFi. Concomitant MTX (57%), oral corticosteroids (17%), and NSAIDs (64%) were permitted. Patients were randomized to subcutaneous (SC) GUS 100 mg at week (W) 0, W4, then every 8 weeks (Q8W); GUS 100 mg Q4W; or placebo (PBO). At W24, PBO patients were switched to GUS 100 mg Q4W. In VOYAGE 1&2, in which concomitant MTX use was prohibited, 1245 patients with moderate to severe PsO were treated and randomized to SC GUS 100 mg at W0, W4, W12, then Q8W; or PBO at W0, W4, W12, with crossover to GUS at W16, W20, then Q8W. Adverse events (AEs) and laboratory parameters, analyzed by National Cancer Institute-Common Terminology Criteria for AEs [NCI-CTCAE] toxicity grades, were summarized through the PBO-controlled periods and 1 year.
Results: Safety profiles were generally consistent across the GUS PsO and PsA clinical programs (Table). Time-adjusted incidence rates for numbers of AEs, serious AEs, serious infections, malignancy, major adverse cardiovascular events and AEs leading to discontinuation were generally similar between PsO and PsA. No cases of anaphylaxis or opportunistic infections were reported. Proportions of patients with decreased neutrophil counts and elevations in hepatic transaminases were slightly higher in PsA versus PsO. These abnormalities were mostly of NCI-CTCAE Grade 1 or 2 (< lower limit of normal-1000/mm3 for neutrophils; < 5.0 x upper limit of normal for aspartate transaminase/alanine aminotransferase [AST/ALT]), generally transient, required no medical interventions, resolved spontaneously, and did not lead to interruption or discontinuation of treatment. Through 1 year, proportions of patients with ALT/AST elevations in PsA trials were slightly higher for GUS Q4W than Q8W and in patients with versus without baseline MTX use.
Conclusion: The GUS safety profile was generally consistent in PsA and PsO GUS-treated patients through 1 year of the DISCOVER and VOYAGE trials.
To cite this abstract in AMA style:
Rahman P, Gottlieb A, Merola J, Armstrong A, Langley R, Lebwohl M, Griffiths C, Shawi M, Yang Y, Hsia E, Kollmeier A, Xu X, Izutsu M, Ramachandran P, Sheng S, You Y, Miller M, Ritchlin C, McInnes I. Comparable Safety Profile of Guselkumab in Psoriatic Arthritis and Psoriasis: Results from Phase 3 Trials Through 1 Year [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/comparable-safety-profile-of-guselkumab-in-psoriatic-arthritis-and-psoriasis-results-from-phase-3-trials-through-1-year/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparable-safety-profile-of-guselkumab-in-psoriatic-arthritis-and-psoriasis-results-from-phase-3-trials-through-1-year/