Session Information
Date: Friday, November 6, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Psoriatic arthritis (PsA) is associated with a high burden of disease and an increased risk of comorbidities. Recent data suggest that patients with moderate PsA benefit most from apremilast (APR) treatment (Mease PJ, et al. Arthritis Care Res [Hoboken]. 2020 Jan 7. [Epub ahead of print]). Results from an earlier analysis of the REWARD study suggest that patients with limited joint involvement may benefit from APR treatment, with improvements in the perceived impact of disease (Jansen TL, et al. Ann Rheum Dis. 2019;78:913 [abstract FRI0442]). Patients with limited joint involvement or comorbidities are underrepresented in randomised controlled trials; therefore, evidence from real-world patient cohorts is needed to assess and compare the impact and burden of disease on patients with limited vs. extensive joints who may also have comorbidities. This study compared the burden of disease and comorbidities in patients with PsA who have limited joint involvement with patients with PsA who have extensive joint involvement.
Methods: The prospective, multicenter, observational REWARD study assessed the impact of using the Psoriatic Arthritis Impact of Disease (PsAID) questionnaire (score range: 0-10), presence of domains of PsA (enthesitis, dactylitis, skin psoriasis, nail psoriasis, axial involvement), and ongoing or history of comorbidities of interest on patients with PsA considered for apremilast treatment in The Netherlands. This interim analysis compared results in patients with limited joint involvement (swollen joint count [SJC] ≤4) vs. more extensive joint involvement (SJC >4).
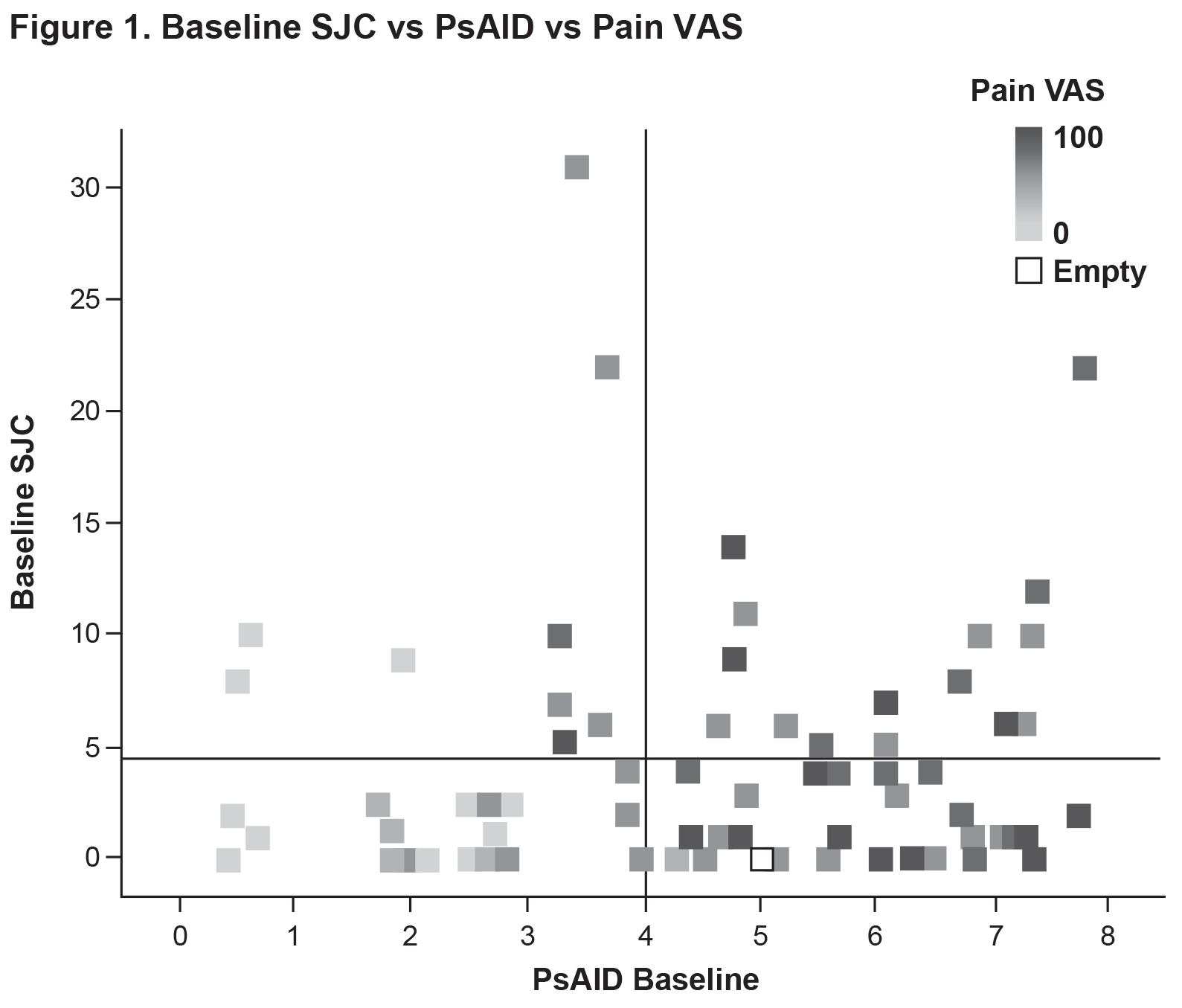
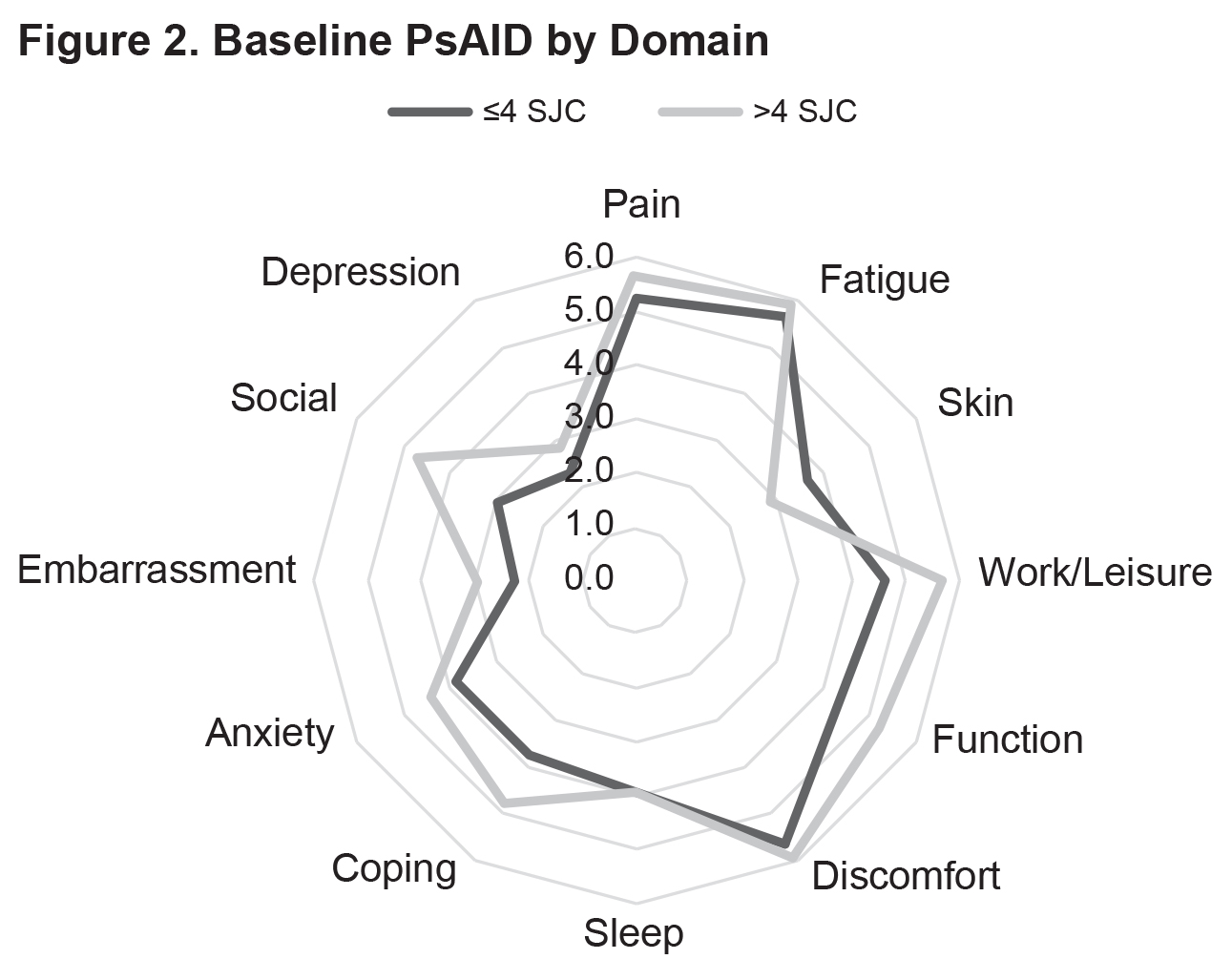
Results: Currently, 77 patients have been included in the analysis (SJC ≤4: n=53; SJC >4: n=24) (Table 1). Mean baseline PsAID scores were 4.4 vs. 4.8 for the SJC ≤4 vs. SJC >4 groups (Figure 1). The proportions of patients who were not in the PsAID-defined Patient Acceptable Symptom State (PASS) were 58.7% for the SJC ≤4 group and 62.5% for the SJC >4 group. Mean pain visual analog scale (VAS) scores (0-100 mm) were 45.9 vs. 53.4 for the SJC ≤4 group vs. for the SJC >4 group. Mean scores for the individual PsAID domains for the SJC ≤4 vs. SJC >4 groups were generally comparable (Figure 2). Presence of specific manifestations of PsA for patients in the SJC ≤4 group vs. the SJC >4 group, respectively, were: moderate to severe psoriasis (psoriasis-involved body surface area [BSA] >3: 31.4% vs. 21.7%), nail psoriasis (45.3% vs. 41.7%), enthesitis (Leeds Enthesitis Index >0: 43.4% vs. 45.8%), dactylitis (18.9% vs. 33.3%), and axial involvement (3.8% vs 8.3%). Comorbidities in ≥5% of either group (SJC ≤4 vs. SJC >4) included hypertension (30.2% vs. 37.5%), hypercholesterolemia (13.2% vs. 16.7%), uveitis (1.9% vs. 8.3%), malignancy (0.0% vs. 8.3%), heart failure (5.7% vs. 8.3%), and depression (5.7 vs. 4.2%).
Conclusion: In this real-world study, no strong associations between SJC and patient-reported impact of disease or pain were observed. Similar to patients with more extensive joints involvement, patients with limited joint involvement had an associated substantial burden of disease, with more than half not achieving PsAID PASS.
To cite this abstract in AMA style:
Jansen T, van Vliet A, Vis M. Comparable Impact and Burden of Disease of Psoriatic Arthritis Patients with Limited Joint Involvement vs. Those with More Extensive Joint Involvement: Interim Results from a Prospective, Multicenter, Real-World Study in Patients Treated with Apremilast [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/comparable-impact-and-burden-of-disease-of-psoriatic-arthritis-patients-with-limited-joint-involvement-vs-those-with-more-extensive-joint-involvement-interim-results-from-a-prospective-multicenter/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparable-impact-and-burden-of-disease-of-psoriatic-arthritis-patients-with-limited-joint-involvement-vs-those-with-more-extensive-joint-involvement-interim-results-from-a-prospective-multicenter/