Session Information
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Immune checkpoint inhibitors (ICIs) are revolutionizing the treatment of some advanced cancers. Gut microbiota has emerged as an important component of anti-tumoral response and can also be related to the occurrence of immune-related adverse events (irAEs). It has recently been shown that antibiotic treatment given at the initiation of ICI therapy had a dramatic impact on microbiota that compromised the anti-tumoral effect of ICIs.
The objective of this study is to evaluate whether co-medications known to have a potential impact on gut microbiota may alter ICI efficacy and/or irAE occurrence when given at ICI onset.
Methods: This was a retrospective cohort study including all cancer patients who received ICIs at our institution from May 2015 to September 2017. Co-medications given to the patients within one month before or one month after the first administration of ICI were extracted from medical records on the basis of a predefined list of medications known to impact gut microbiota. The tumour response, occurrence of irAEs and patient outcomes were assessed on a regular basis. Overall survival (OS) has been considered from the start of ICI therapy.
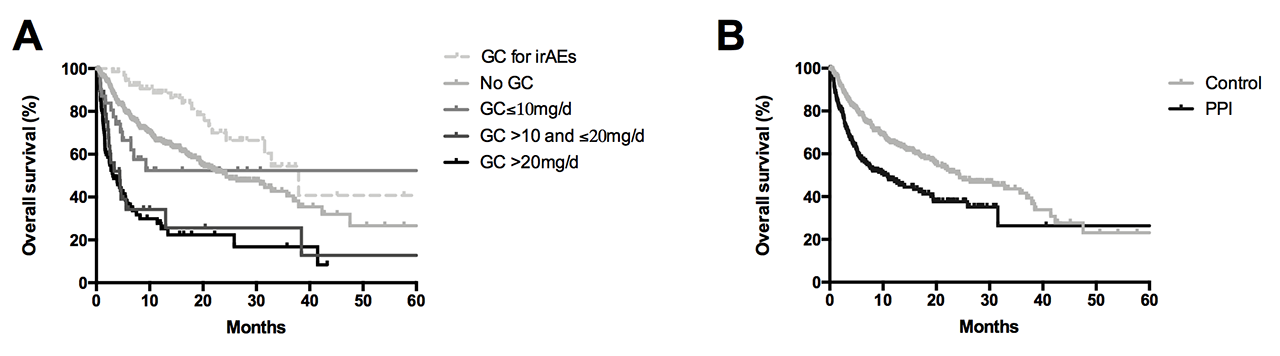
Results: 635 patients (70% male, mean age 64.5 years) were included, of whom 293 had melanoma, 150 had advanced non-small cell lung cancer and 83 had renal carcinoma. A previous autoimmune disorder was present in 8% of patients, mainly rheumatic and endocrine diseases. Psychotropic drugs (41.1%), proton pump inhibitors (PPIs) (37.3%), angiotensin-converting enzyme (ACE) inhibitors and/or angiotensin II receptor blockers (ARBs) (32%), glucocorticoids (GC) (24.2%), antibiotics (21.4%), statins (20.8%) and morphine (20.6%) were the most co-prescribed medications. Baseline GC use, when ≥ 10mg of prednisone equivalent, was associated with significant decreased OS (median 4.5 months versus 24.3 months; p< 0.0001) and a less frequent tumour response (55% versus 73% ; p=0.0001). When given after ICI onset for the management of irAEs, GC did not influence ICI efficacy (Figure A). Baseline PPI use also altered both OS (median 10.9 versus 24.3 months; p< 0.0001) and tumour response (62% versus 71%; p=0.02) (Figure B). We confirmed the detrimental impact of antibiotics when given at ICI onset, and also found worse outcomes for patients receiving baseline psychotropic drugs (median OS 9.3 versus 19.4 months; p=0.0001). No significant difference was observed with baseline use of NSAIDs, aspirin, statins and ARBs/ACE. Furthermore, co-medication with antibiotics, GC, PPIs, morphine, NSAIDs, aspirin and psychotropic drugs was associated with decreased occurrence of irAE.
Conclusion: As many of these treatments are used by rheumatologists, one should be aware of their potential detrimental effect when used at ICI initiation, that sometimes could have been avoided.
To cite this abstract in AMA style:
Kostine M, Mauric E, Barnetche T, Rouxel L, Dutriaux C, Dousset L, Prey S, Beylot-Barry M, Seneschal J, Veillon R, Charlotte V, Daste A, Charlotte D, Sionneau B, Gross-Goupil M, Ravaud A, Forcade E, Bannwarth B, Mehsen-Cetre N, Truchetet M, Richez C, Schaeverbeke T. Commonly Used Drugs in Rheumatology May Alter Anti-Tumoral Response to Immune Checkpoint Inhibitors [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/commonly-used-drugs-in-rheumatology-may-alter-anti-tumoral-response-to-immune-checkpoint-inhibitors/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/commonly-used-drugs-in-rheumatology-may-alter-anti-tumoral-response-to-immune-checkpoint-inhibitors/