Session Information
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose:
Systemic sclerosis (SSc), a multi-organ fibrotic disease, affects the gastrointestinal tract (GIT) in 90% of patients. An urgent unmet need in the management of SSc GIT involvement is the availability of non-invasive investigations for early diagnosis and monitoring. Development of a new MRI sequence, T1 MOLLI (modified look-locker inversion recovery) mapping, has been applied to cardiac imaging, which enables detection and quantification of diffuse fibrosis without contrast. In this pilot study comparing SSc patients with healthy controls, we investigate FDG-PET/MRI in diagnosing SSc GIT involvement, hypothesizing that (i) MRI T1 MOLLI values, denoting fibrosis, is higher in SSc patients than healthy controls, and (ii) PET detects bowel inflammation associated with early mild GIT involvement.
Methods:
Ten patients fulfilling the 2013 ACR/EULAR criteria for SSc and 10 healthy age and sex matched controls underwent scanning. SSc patients either had early SSc <3 years and asymptomatic to mild GIT symptoms (Group A, n=5), or moderate to severe GIT symptoms regardless of disease duration (Group B, n=5), as determined by a validated questionnaire (Table 1). All subjects fasted 6 hours prior and had non-spicy low-residue diet 3 days prior. SSc patients underwent PET-MRI scan, 60 minutes after injection of 6mCi FDG tracer and immediately after injecting 10mg hyoscine butylbromide (reduce peristalsis). Breath-hold native T1 MOLLI mapping was acquired. Controls underwent the same MRI protocol. FDG uptake was quantified by specific uptake value (SUV). Student t-test and box plots were used to evaluate statistical significance (p<0.05).
Results:
Majority were females with a mean age of 48 years. Mean T1 values for the large and small bowels were higher in SSc patients than healthy controls (large bowel: 1206±148ms vs 869±192ms respectively, p<0.001; small bowel: 1425±245ms vs 1150±115ms respectively, p=0.005) (Table 1).
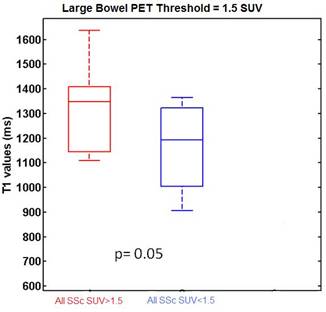
All SSc patients had high PET uptake in the bowel (on average 25% of their total bowel had SUV >1.5). PET SUV values in the large and small bowels were higher in Group A (mean GIT score 0.11) than Group B (mean GIT score 0.57) patients. In the large bowel, T1 values were higher in the regions of inflamed bowel, as defined by PET SUV >1.5 (Figure 1).
Conclusion:
Our preliminary results suggest the feasibility of using T1 MOLLI to evaluate bowel fibrosis. FDG-PET showed uptake in all SSc cases, with increased bowel inflammation occuring in patients with early mild disease. FDG-PET/MRI is potentially a useful diagnostic and monitoring tool for SSc GIT disease.
Table 1
| Group A (n=5) | Group B (n=5) | All SSc patients (n=10) | Controls (n=10) | |
| Demographic and clinical features | ||||
| Female sex, n (%) | 5 (100%) | 4 (80%) | 9 (90%) | 9 (90%) |
| Age, years | 42.4 ± 15.9 | 54.0 ± 5.7 | 48.2 ± 12.8 | 48.0 ± 12.7 |
| Limited/Diffuse SSc, n | 2/3 | 5/0 | 7/3 | Not applicable |
| Mean disease duration from Raynaud’s phenomenon onset, years | 2.6 ± 0.8 | 5.0 ± 4.3 | 3.8 ± 3.2 | Not applicable |
| Mean disease duration from non-Raynaud’s phenomenon onset, years | 3.1 ± 1.0 | 4.7 ± 3.6 | 3.9 ± 2.6 | Not applicable |
| Total GIT score | 0.11 ± 0.07 | 0.57 ± 0.29 | 0.34 ± 0.32 | 0 |
| T1 MOLLI and PET findings | ||||
| T1 (large bowel) (ms) | 1218 ± 142 ¶ | 1193 ± 170 ¶ | 1206 ± 148 ¶ | 869 ± 192 |
| T1 (small bowel) (ms) | 1335 ± 112 ¶ | 1516 ± 320 ¶ | 1425 ± 245 ¶ | 1150 ± 115 |
| PET SUV (large bowel) | 1.31 ± 0.30 | 1.11 ± 0.19 | 1.21 ± 0.26 | Not applicable |
| PET SUV (small bowel) | 1.31 ± 0.35 | 1.19 ± 0.33 | 1.25 ±0.33 | Not applicable |
| Total GIT: Total Gastrointestinal Tract (version 2.0) score, from University of California Los Angeles Scleroderma Clinical Trials Consortium ¶ p < 0.05 versus controls | ||||
Figure 1
To cite this abstract in AMA style:
Ng SA, Marchesseau S, Wang YT, Schaefferkoetter J, Xie W, Ng D, Totman J, Low AH. Combined Positron Emission Tomography and Magnetic Resonance Imaging in Assessing Gastrointestinal Involvement in Systemic Sclerosis: A Pilot Study [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/combined-positron-emission-tomography-and-magnetic-resonance-imaging-in-assessing-gastrointestinal-involvement-in-systemic-sclerosis-a-pilot-study/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/combined-positron-emission-tomography-and-magnetic-resonance-imaging-in-assessing-gastrointestinal-involvement-in-systemic-sclerosis-a-pilot-study/