Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The mechanistic target of rapamycin (mTOR) pathway and glutamine metabolism are activated cooperatively in the differentiation and the activation of inflammatory immune cells such as effector lymphocytes and M1 macrophages. Myeloid-derived suppressor cells (MDSCs) are an immature myeloid cell population with immunosuppressive ability. The promotion of the expansion and immunosuppressive ability of MDSCs by mTOR inhibition has been reported. The aim of this study is to elucidate the effect of combined inhibition of mTOR and glutamine metabolism on immune cells and therapeutic effect in a mouse model of rheumatoid arthritis.
Methods: The proliferation of CD4+ T cells treated with four patterns of drugs; 1) DMSO (control), 2) rapamycin (Rapa), 3) 6-Diazo-5-oxo-L-norleucine (DON; a glutamine antagonist), or 4) the combination of rapamycin and DON (Rapa+DON), were assessed by CFSE-dilution assay. The differentiation of CD4+ T cells and bone marrow cells from untreated Balb/c mice were analyzed by flow cytometry. Immunosuppressive ability of in vitro generated-MDSCs were assessed by co-culture with CFSE-labeled CD4+ T cells. The four patterns of drugs were administered intraperitoneally to arthritic SKG mice and splenocytes were analyzed by flow cytometry.
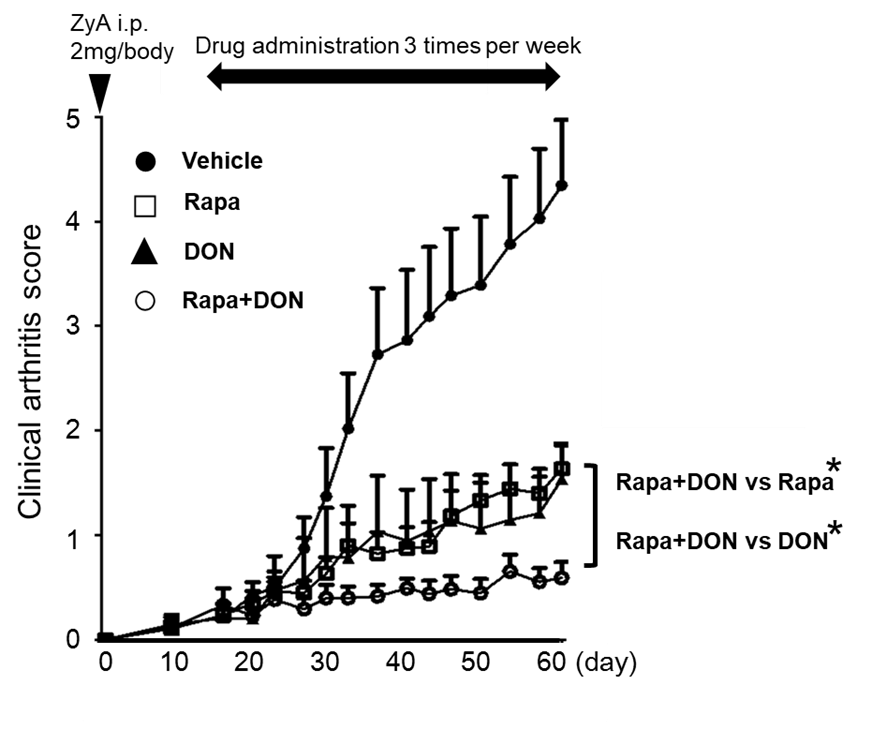
Results: Rapamycin and DON synergistically inhibited the CD4+ T cell proliferation and both of them inhibited the Th17 cell differentiation in vitro. DON significantly suppressed the differentiation of dendritic cells and macrophages, and increased the proportions of MDSCs in vitro. Most of in vitro generated-MDSCs treated with rapamycin or DON were Ly6G+ granulocytic (G)-MDSCs. On the other hand, in vitro-generated G-MDSCs treated with rapamycin suppressed the proliferation of CD4+ T cells more strongly than G-MDSCs treated without rapamycin. The combination of rapamycin and DON synergistically suppressed arthritis in SKG mice in vivo (see Figure). The number of CD4+ T cells in splenocytes was the most suppressed in the combination therapy group and the proportions of Th17 cell were suppressed in rapamycin, DON, or the combination therapy groups.
Conclusion: The combination of rapamycin and DON synergistically ameliorated arthritis in SKG mice possibly through suppressing CD4+ T cell proliferation and Th17 differentiation.
To cite this abstract in AMA style:
Ueda Y, Saegusa J, Okano T, Sendo S, Yamada H, Akashi K, Onishi A, Morinobu A. Combined Inhibition of Mechanistic Target of Rapamycin and Glutamine Metabolism Inhibits CD4 T Cell Proliferation and Th17 Differentiation, Facilitates the Expansion of Myeloid-Derived Suppressor Cells, and Synergistically Ameliorates Arthritis in SKG Mice [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/combined-inhibition-of-mechanistic-target-of-rapamycin-and-glutamine-metabolism-inhibits-cd4-t-cell-proliferation-and-th17-differentiation-facilitates-the-expansion-of-myeloid-derived-suppressor-cell/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/combined-inhibition-of-mechanistic-target-of-rapamycin-and-glutamine-metabolism-inhibits-cd4-t-cell-proliferation-and-th17-differentiation-facilitates-the-expansion-of-myeloid-derived-suppressor-cell/