Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Combined biological or targeted therapy (CBTT) is rarely considered in clinical practice due to contraindication in guidelines, potential safety concerns and high costs. However, previous successful experiences in inflammatory bowel disease (IBD) and our own early data in selected refractory spondyloarthritis (SpA) have shown encouraging results1. Our aim was to explore effectiveness and safety of CBTT in patients with SpA in a real-world setting.
Methods: This a retrospective and multicenter study. We identified patients with SpA and simultaneous combined use of 2 biological or targeted (JAK inhibitors) agents with different therapeutic targets, with a minimum exposure of three months. All patients fulfilled axial or peripheral SpA according to ASAS criteria and provided a written informed consent for off-label use of CBTT. Demographic, clinical, laboratory, and safety data were collected from electronic medical records. Effectiveness was assessed using ASDAS-CRP, DAPSA and DAS-28-CRP indices. Major clinical improvement (MCI) was defined as a change in ASDAS-CRP >2 or DAS-28-CRP >1.2 units or improvement of >85% in DAPSA. Data analysis included descriptive statistics for categorical and continuous variables and between-groups comparisons were performed using t-student test or chi-squared test.
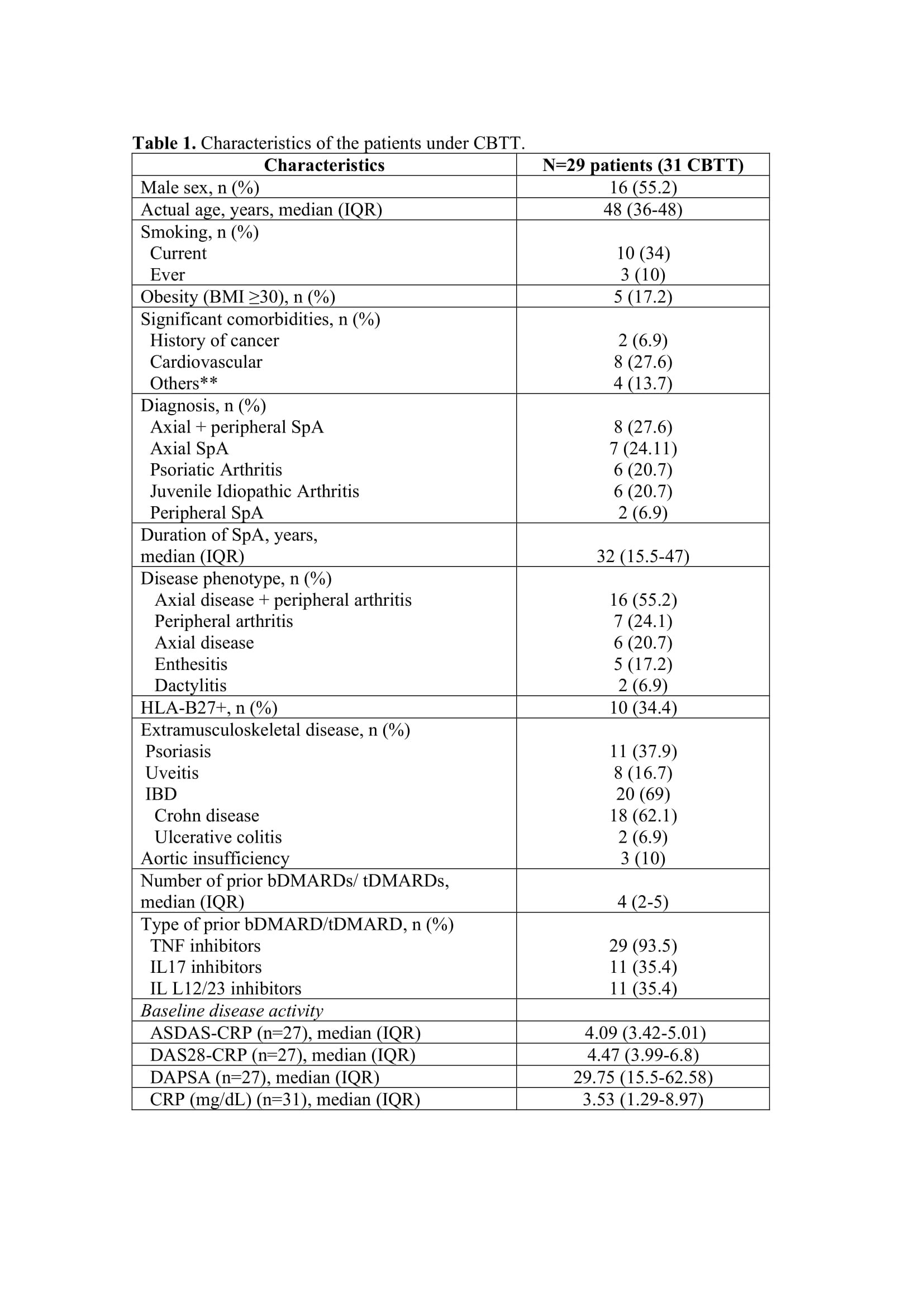
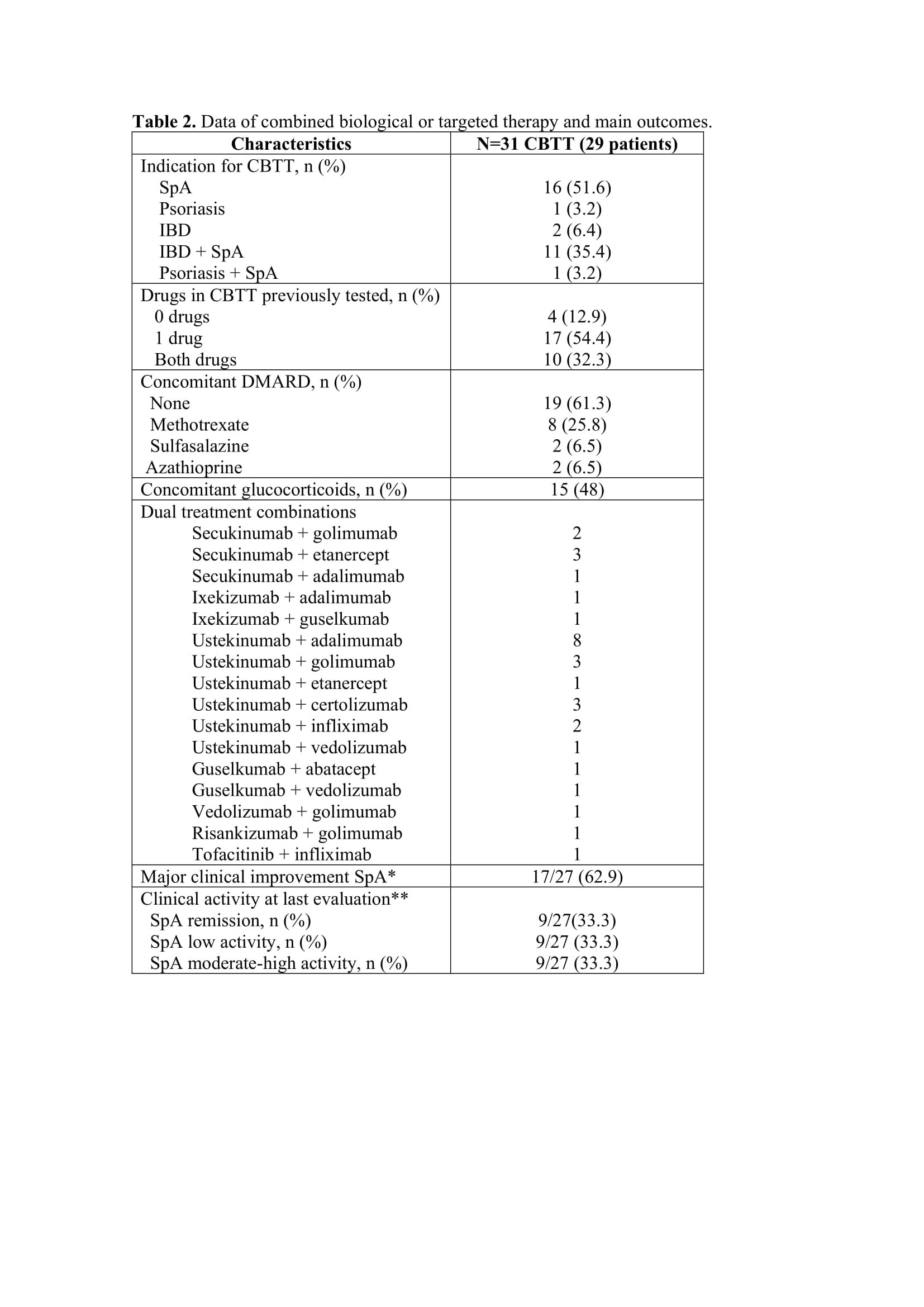
Results: A total of 31 CBTT courses were identified in 29 SpA patients, with concomitant IBD in 20 (69%) (Table 1). The main indications for CBTT were active musculoskeletal and bowel disease (Table 2). Three patients in 4 courses presented remission or low SpA activity at baseline and were excluded in the efficacy analysis. Most patients (87%) had previously failed at least one of the two therapies used in CBTT. The most common combination was TNF inhibitor plus IL12/23 inhibitor agent (n=17; 54%) or an IL17 inhibitor (n=7; 22.5%). Just one combination included a JAK inhibitor.
Median exposure to CBTT was 12 months (IQR 6-18). At final evaluation, we found a significant mean reduction in ASDAS-CRP (1.84; 95% CI: 1.21-2.47; p=0.001). MCI and remission/low activity rates were 62.9% (n=17) and 66.7% (n=18), respectively.
In a bivariate analysis, the presence of HLA-B27 was associated to MCI (OR 2.12; p=0.01) whereas the presence of axial or peripheral domain (p=0.711) and diagnosis (p=0.617) were not (Table 1). The limited sample size prevented us from performing stratified analysis or adjusting multivariate models.
Twelve patients (37.8%) discontinued CBTT: 7 for inefficacy (5 with IBD activity), 2 for adverse events (AE) and 3 patients due to other causes. In 31 courses, 2 serious AE were identified under CBTT (non-infectious diffuse pulmonary infiltrates and a staphylococcal bacteriemia).
Conclusion: Our preliminary results suggest that CBTT might be a reasonable therapeutic alternative in selected refractory SpA patients, with acceptable effectiveness/safety ratio. Prospective wider studies are warranted to confirm these data.
References
1. Valero C et al. ARD. 2022 Jun;81(6):899-901.
**Liver disease, depression, chronic kidney disease, chronic respiratory disease (one of each).
*In patients with moderate-high baseline SpA activity, major clinical improvement was defined as a change in ASDAS-CRP>2 and DAS_28-CRP>1.2 units and improvement of >85% in DAPSA.
**Cut-offs for remission and low activity criteria for axial and peripheral disease were ASDAS-CRP <1.3/<2.1 or DAS_28-CRP<2.6/<3.2 or DAPSA<4/<14, respectively.
To cite this abstract in AMA style:
Valero-Martinez c, Font Urguelles J, Sallés M, JOVEN B, Ramirez Garcia F, Juanola X, Almodóvar R, Laiz a, Moreno M, Pujol M, Sala-Icardo L, Castañeda S, Garcia-Vicuña R. Combined Biological or Targeted Therapy in Spondyloarthritis: Experience from a Multicenter Case Series in Spain [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/combined-biological-or-targeted-therapy-in-spondyloarthritis-experience-from-a-multicenter-case-series-in-spain/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/combined-biological-or-targeted-therapy-in-spondyloarthritis-experience-from-a-multicenter-case-series-in-spain/