Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Measuring improvement or worsening is problematic in lupus trials. The British Isles Lupus Assessment Group (BILAG) index often fails to capture sustained decrease in disease activity when the change levels off. The SLE Disease Activity Index (SLEDAI) is inflexible if improvement does not meet a low threshold definition. False positive or negative flares create additional problems. “Mild/moderate” worsening on the SLEDAI Flare Index (SFI) can be due to insignificant changes. According to an older BILAG flare definition ( >/= 2 new B or 1 new A), which is still commonly used, a false flare occurs if previously improving disease stabilizes, while true worsening is frequently missed when organ scores remain unchanged. A newer BILAG flare definition addresses most of these issues, but fails to account for > 1 feature flaring in a single organ or to allow a moderately severe flare in only 1 organ to rate more than “mild.” Given the pitfalls of glossary-based measures, we sought the advice of the clinician in determining changes in disease severity.
Methods: A simple algorithm for clinician’s global impression of change (CGIC) was tested during a phase 2 trial of the B Cell modulator, Xmab5871. investigators were asked to rate disease progress at each visit as “no change or insignificant change” (NC) “significant partial improvement” (PI), “major or complete improvement” (MI), “significant moderate worsening” (MW), or “severe worsening” (SW). Discrepancies between the CGIC and other instruments were brought to the investigators’ attention, but it was emphasized that the clinician’s opinion could conflict with the technical scoring. Results were collected in an online database along with the BILAG, SLEDAI, PGA and SFI results.
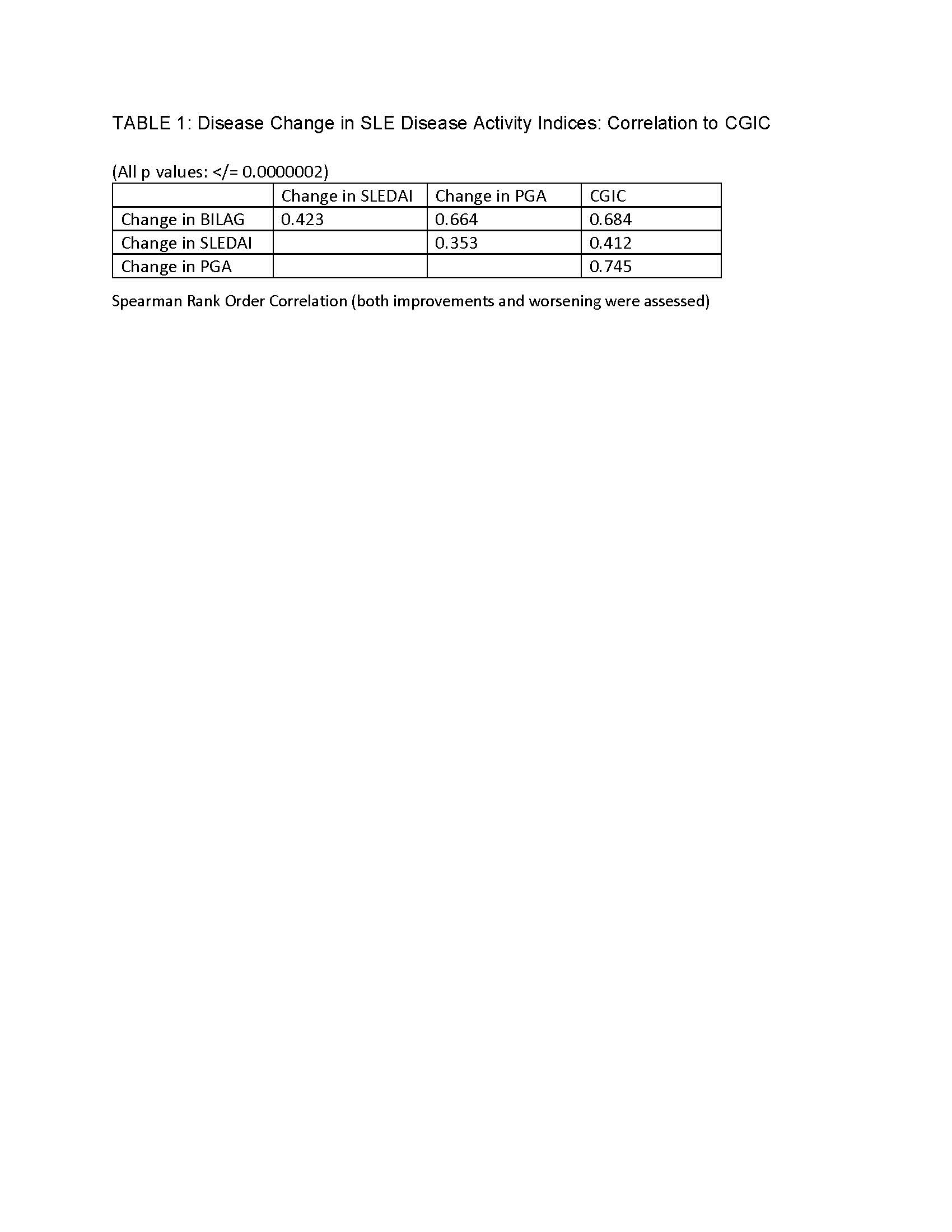
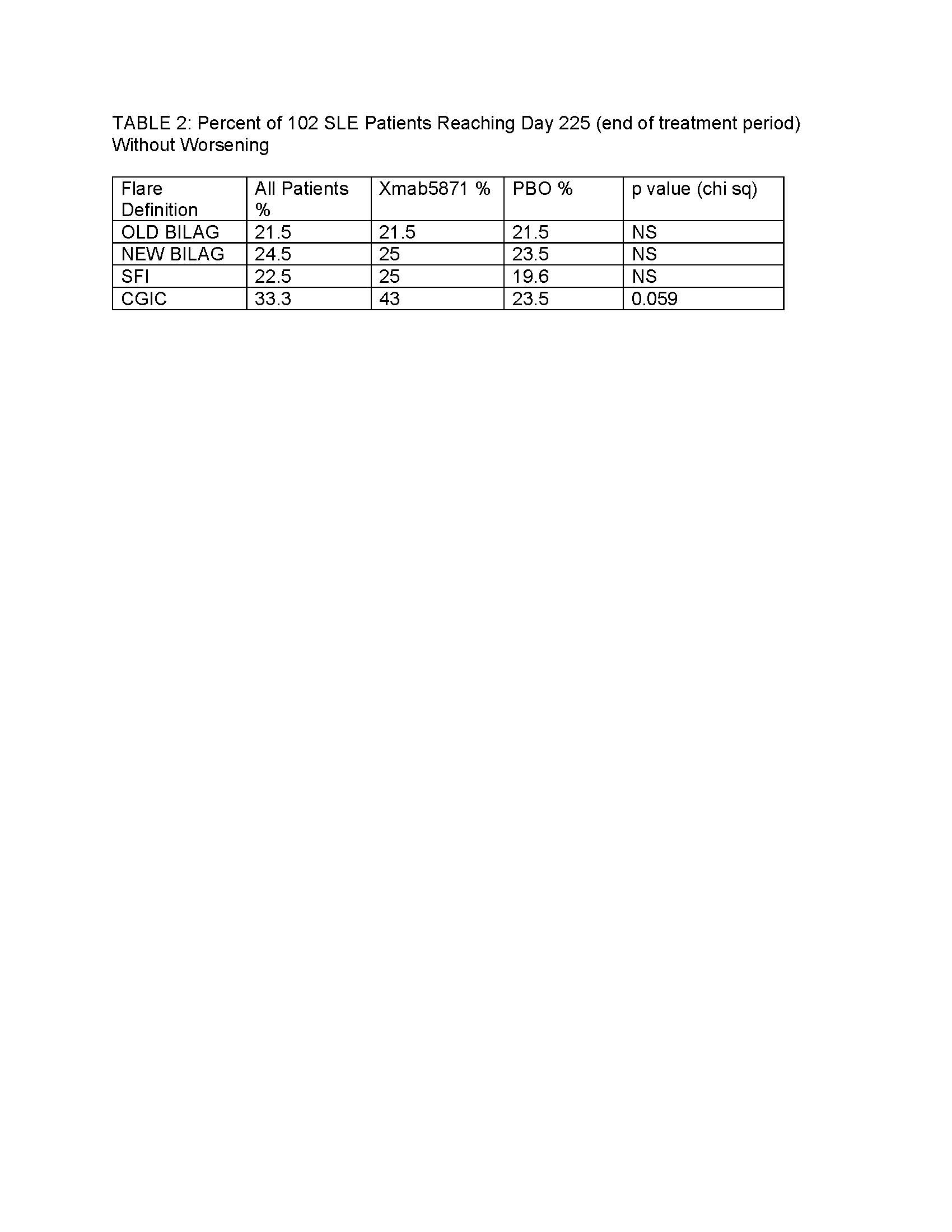
Results: Of 104 randomized patients, data from 102 were available from 2-11 visits. The results of the trial have been reported elsewhere; briefly, the primary endpoint, maintenance of improvement, was met by 42% of XmAb5871-treated patients vs 28.6% of the placebo (PBO) group (p=0.18) and time to flare was longer in the XmAb5871 group (p=0.025). Using the CGIC, clinicians rated 445 visits as NC, 148 PI, 64 MI, 84 MW and 8 SW. CGIC was tested as a gold standard for the comparison of other measures of change that are used in SLE trials. Results of this study are summarized in Tables 1 and 2.
Conclusion: Clinician’s opinion, recorded using CGIC, is better reflected by changes of BILAG and PGA than it is by the SLEDAI, which offers fewer gradations of scoring. More patients were free of flare using the CGIC compared to other instruments. In the double-blind trial of Xmab5871 the increased CGIC threshold for defining worsening disease impacted results for patients receiving active treatment more than PBO, suggesting the utility of CGIC as a gold standard for clinical significance.
To cite this abstract in AMA style:
Askanase A, Saxena A, Thanou A, Arriens C, Zack D, Merrill J. Clinician’s Simple Opinion of SLE Disease Progress: Used in a Clinical Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/clinicians-simple-opinion-of-sle-disease-progress-used-in-a-clinical-trial/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinicians-simple-opinion-of-sle-disease-progress-used-in-a-clinical-trial/