Session Information
Date: Monday, November 11, 2019
Title: 4M096: Spondyloarthritis Including Psoriatic Arthritis – Clinical III: Miscellaneous (1818–1823)
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Physical function in axial spondyloarthritis (axSpA) usually assessed by the BASFI questionnaire is an established core domain of that disease. There is evidence that self- reported physical function is not equivalent with the actual performance of patients. Physical performance can be assessed as a single task such as grip strength or single stance, or as a generic compound measure such as the short physical performance battery test (SPPB). SPPB comprises a chair rising test, a balance test and gait speed. The aim of the study is to investigate which performance tests are most frequently impaired in patients with axSpA.
Methods: Consecutive axSpA patients presenting to our tertiary hospital underwent a standardized assessment including patient and disease characteristics, patient-reported outcomes (ASDAS, BASFI, BASMI, ASAS Health Index (ASAS HI), PHQ-9) and performance tests (SPPB, grip strength and single stance). Structural damage was assessed by mSASSS. Validated cut-offs were used for SPPB, chair rise test, grip strength and gait speed. Impairment of performance tests as well as discrimination between subgroups was analysed.
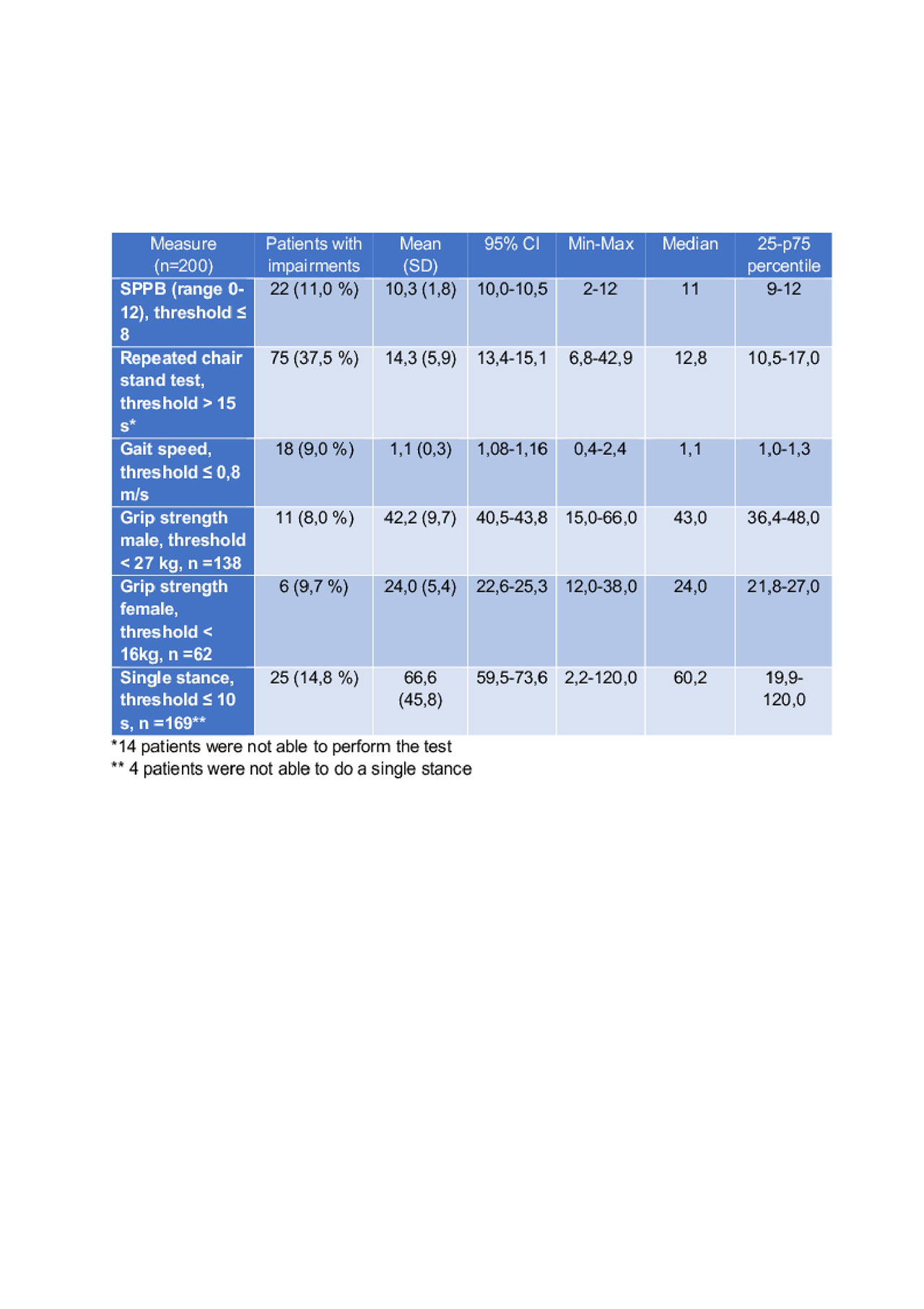
Results: A total of 200 patients (r-axSpA 65.5%, nr-axSpA 34.5%) were included: 69% males, 44.3±12.5 years of age, mean symptom duration 17.9±12.6 years, mean ASDAS 2.5±1.1, BASFI 4.0±2.7, BASMI 3.5±1.8, ASAS HI 7.0±4.1, PHQ-9 8.8±6.2, mSASSS (n=157) 10.2± 18.8. A total of 132 patients were treated with bDMARDs (66.5%). The two most impaired performance tests were repeated chair rising and single stance (Figure 1). An impairment in≥ 1 performance test was seen in 87 patients (43.5%). Patients with impairments, in comparison to those without, were older (48.9 vs. 40.8 years), more often obese (28.7 vs. 26.1%), more often depressed (PHQ 12.1 vs. 6.3%), had lower BASFI values (5.7 vs. 2.8), a decreased ASAS HI (9.6 vs. 5.0), and higher disease activity (ASDAS 3.0 vs. 2.1), all p< 0.01. The documented impairment in performance was irrespective of medication and structural damage on the group and the individual patient level. The correlation between BASFI and the performance test was moderate for SPPB (0.6), gait speed (0.5), chair rise (0.5) and single stance (0.4), while the correlation between BASFI and grip strength (0.2) and mSASSS (0.2) was rather limited.
Conclusion: n this consecutively recruited relatively young axSpA patients with limitations in physical function and health as assessed by established measures, we found a high prevalence of patients who didn’t perform well in tests originally developed for older people. Importantly, a lot of impairment was seen when patients were asked to perform complex tasks requiring coordination and muscle strength. Impairment was present even though most patients received bDMARDs. Since such impairment is potentially influenced by physiotherapeutic interventions, we propose to perform studies to address these deficits. Our data strongly suggest to not only collect questionnaires but also do performance tests to better assess the ‘real’ physical capacity of patients.
To cite this abstract in AMA style:
Kiltz U, Ahomaa E, Bühring B, Baraliakos X, Braun J. Clinically Relevant Deficits in Performance Tests in Patients with Axial Spondyloarthritis(axSpA) – Collecting Questionnaires Is Insufficient [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/clinically-relevant-deficits-in-performance-tests-in-patients-with-axial-spondyloarthritisaxspa-collecting-questionnaires-is-insufficient/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinically-relevant-deficits-in-performance-tests-in-patients-with-axial-spondyloarthritisaxspa-collecting-questionnaires-is-insufficient/