Session Information
Date: Sunday, November 5, 2017
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose:
Recently, our group conceived a risk score for clinical manifestations of APS [the global APS score or GAPSS] that takes into account the combination of independent cardiovascular risk factors and the aPL positivity profile. These include hyperlipidemia, arterial hypertension, aCL, anti-b2GPI, aPS/PT and the LA. A complementary version, the adjusted GAPSS or aGAPSS, which excludes aPS/PT, was also designed.
Methods:
We pooled data from available cohort studies, including a total of 10 studies, counting for a total of 2273 patients in which the GAPSS score has been applied. A search strategy was developed a priori to identify available cohort that reported findings that investigated the clinical utility of GAPSS or aGAPSS score.
Results:
When pooling together available data, GAPSS/aGAPSS was applied in a total of 2273 patients. Studies characteristics and patients enrolled are summarized in Table 1.
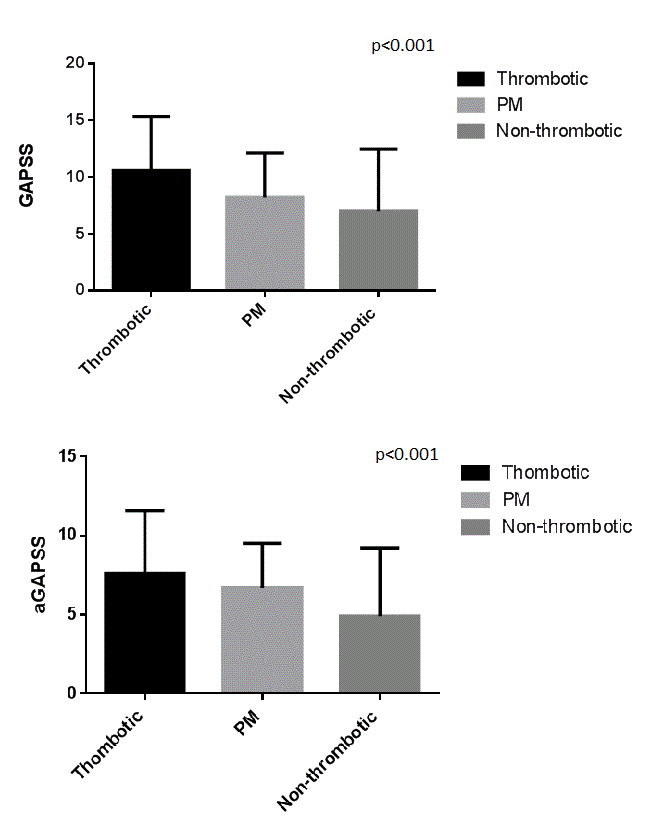
Seven studies used the GAPSS in their cohort, whether three studies used the aGAPSS. In brief, we found a statistically significant difference in the cumulative GAPSS and aGAPSS scores between patients that experienced arterial and/or venous thrombotic event (Cumulative GAPSS 10.6±4.74 and aGAPSS 7.6±3.95), patients without any thrombotic manifestation (Cumulative GAPSS 7.01±5.46 and aGAPSS 4.9±4.33) and patients with pregnancy morbidity (Cumulative GAPSS 8.79±2.59 and aGAPSS 6.7±2.8).
The highest levels of GAPSS were found in patients that experienced arterial thrombosis (mean GAPSS 12.2±5.2) and patients that experienced any recurrences of clinical manifestations of APS (mean GAPSS 13.7±3.1).
Conclusion:
GAPSS may represent a useful tool to assess the thrombosis or pregnancy loss risk in aPL positive patient, switching from the concept of aPL as a sole diagnostic antibody to aPL as risk factors for clinical events. A risk assessment, using appropriate tools as GAPSS, should be implemented to identify and monitor those patients at a higher risk of recurrences and those needing a strict control of all modifiable risk factor for cardiovascular events; in agreement with the above, in the future the management of APS should also modulate according to the GAPSS values.
Table 1. Demographic, clinical and laboratory characteristics of the cohort
|
STUDY |
YEAR |
STUDY DESIGN |
AIM |
NUMBER OF PATIENTS |
PATIENTS’ CHARACTERISTICS |
|
Sciascia et al.
|
2013 |
Cross-Sectional |
To validate the first GAPSS score with a validation cohort |
105 |
SLE |
|
Sciascia et al.
|
2014 |
Prospective |
To prospectively and independently validate GAPSS, with a follow-up of mean 32.94 (SD 12.06) months |
51 |
SLE aPL positive patients |
|
Zuily et al.
|
2015 |
Prospective |
To investigate the validity of the global APS score (GAPSS) to predict thrombosis in patients |
137 |
patients with aPL and/or SLE |
|
Oku et al.
|
2015 |
Retrospective |
To validate the GAPSS independently |
282 |
41 APS (17 PAPS) patients, 88 SLE without APS, 50 rheumatoid arthritis, 16 Sjögren’s syndrome, 21 systemic sclerosis, 10 polymyositis/ dermatomyositis and 56 other autoimmune diseases |
|
Sciascia et al.
|
2015 |
Retrospective |
To evaluate the clinical relevance of the global APS score (GAPSS) in a cohort of primary APS patients |
62 |
PAPS patients |
|
Zigon et al.
|
2016 |
Retrospective |
To evaluate association of different risk factors with thrombosis; and b) to apply GAPSS on a large cohort of unselected Slovenian patients |
585 |
Systemic Autoimmune Diseases |
|
Sciascia et al.
|
2016 |
Retrospective |
To evaluate the clinical utility of the GAPSS with the help of APS ACTION Registry |
550 |
APS Patients |
|
Zu et al.
|
2016 |
Retrospective |
To evaluate the clinical revalence of aGAPSS in a chinese cohort |
89 |
89 APS Patients |
|
Fernandez Mosteirin et al.
|
2017 |
Retrospective |
To independently validate the aGAPSS to predict thrombosis in a cohort of patients with APS and/or autoimmune disease |
319 |
PAPS diagnosed in 130 patients and 89 SAPS patients, and 100 patients with autoimmune disease without APS |
|
Radin et al.
|
2017 |
Retrospective |
To investigate the validity of aGAPSS in young patients with myocardial infarction |
83 |
APS Patients |
Table 2. GAPSS and aGAPSS between groups
|
Sciascia et al. 2013 |
Sciascia et al. 2014 |
Zuily et al. 2015 |
Oku et al. 2015 |
Sciascia et al. 2015 |
Sciascia et al. 2016 |
CUMULATIVE GAPSS mean(±SD) |
Zu et al. 2016 |
Radin et al. 2017 |
Fernandez Mosteirin et al. 2017 |
CUMULATIVE aGAPPS mean(±SD) |
|
|
Total N |
105 |
51 |
137 |
282 |
62 |
550 |
1187 |
98 |
83 |
319 |
500 |
|
Thrombotic N |
37 |
4 |
16 |
N/A |
39 |
N/A |
96 |
53 |
70 |
201 |
324 |
|
GAPSS mean(± SD) |
9.6 (4.8) |
10 (5.4) |
10.88 (5.06) |
N/A |
11.5 (4.6) |
N/A |
10.6 (4.74) |
9.4 (3.2) |
9.2 (5.1) |
6.58 (3.36) |
7.6 (3.95) |
|
Non Thrombotic N |
68 |
47 |
121 |
N/A |
N/A |
N/A |
236 |
N/A |
N/A |
118 |
118 |
|
GAPSS mean(±SD) |
4.9 (5) |
7.13 (5.75) |
8.15 (5.31) |
N/A |
N/A |
N/A |
7.01 (5.46) |
N/A |
N/A |
4.9 (4.33) |
4.9 (4.33) |
|
PM N |
22 |
N/A |
N/A |
11 |
44 |
419 |
496 |
38 |
N/A |
N/A |
38 |
|
GAPSS mean(±SD) |
7.3 (5) |
N/A |
N/A |
4 |
8.7 (3.2) |
9 |
8.78 |
6.7 (2.8) |
N/A |
N/A |
6.7 (2.8) |
Graph 1. Cumulative GAPSS values between groups
To cite this abstract in AMA style:
Sciascia S, Radin M, Sanna G, Cecchi I, Roccatello D, Bertolaccini ML. Clinical Utility of the Global Antiphospholipid Syndrome Score (GAPSS) for Risk Stratification: A Pooled Analysisfrom 2273 Patients [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/clinical-utility-of-the-global-antiphospholipid-syndrome-score-gapss-for-risk-stratification-a-pooled-analysisfrom-2273-patients/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-utility-of-the-global-antiphospholipid-syndrome-score-gapss-for-risk-stratification-a-pooled-analysisfrom-2273-patients/