Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Racial/ethnic minorities, women, and the elderly experience a disproportionate burden of disease in Rheumatoid Arthritis (RA), making it particularly important to examine drug therapies in these populations. Clinical evidence demonstrates that treatment responses to new RA biologic drugs differ significantly by race/ethnicity. Despite a national health agenda to improve the representation of diverse populations in clinical trials, there have been few large-scale analyses examining the diversity of clinical trial participants within rheumatology, and none focusing on RA. Our objective was to perform a comprehensive systematic review of RA randomized controlled trials (RCTs) published within the last 10 years in order to characterize the representation of racial/ethnic minorities, women, and the elderly.
Methods: We identified RA RCTs that were published in PubMed (1/1/2008 to 1/1/2018), searching MEDLINE using “Rheumatoid Arthritis” and “humans” as MeSH terms and “randomized controlled trial” as a publication type. The titles and abstracts for all 1195 identified records were independently screened by two reviewers using a web-based application called Rayyan QCRI (https://rayyan.qcri.org). Full texts of remaining records (n=922) were screened using a process adapted from a previously published systematic review. Logistic regression models were used to assess changes in the proportion of subjects in each racial/ethnic and gender category over time. Chi-squared tests were used to 1) compare the proportion of trial enrollees across racial/ethnic groups to the overall demographics of the U.S. and 2) to compare the proportion of female and male enrollees to the overall age-adjusted gender prevalence of RA.
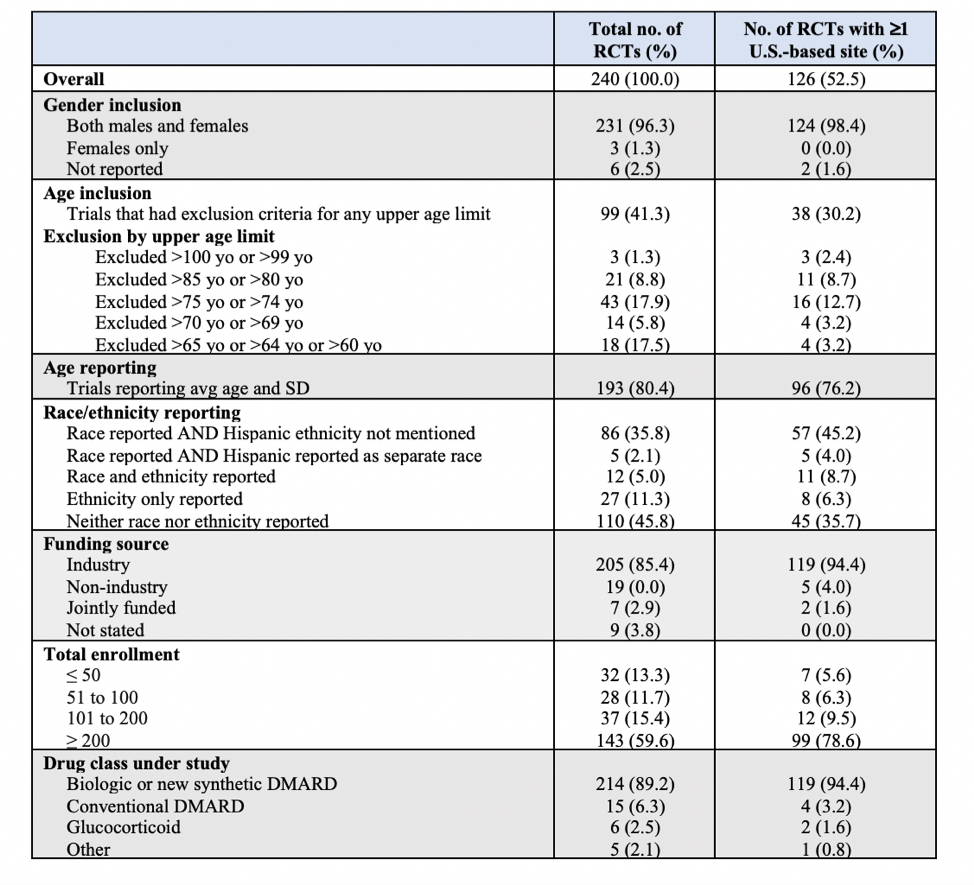
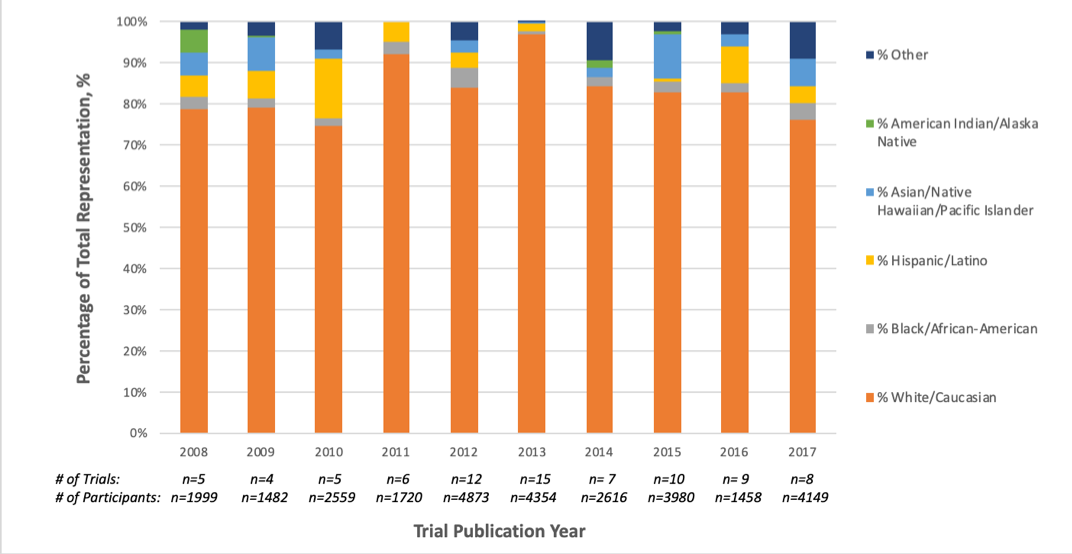
Results: A total of 240 RA RCTs were included in the review (Table 1). The enrollment of minority racial/ethnic groups in U.S.-based RA RCTs was significantly lower than the representation of these groups in the U.S. census population (p< 0.001). While racial and ethnic minorities comprise about 40% of the U.S. population, they only represented 16% of the RCT population for RA. The enrollment of males in RA RCTs with at least one U.S.-based site was significantly lower than the burden of male RA cases nationally (20.4% vs. 28.6%) (p< 0.001). There were no significant changes in the representation of racial/ethnic groups or gender over the observed period (p-trend >0.05) (Figure 1). Reporting of an upper age limit as exclusion criterion was observed in 41.3% (n=99) of the RA RCTs (Table 1).
Conclusion: Despite national efforts to increase clinical trial diversity, there was no trend to improved representation of minority racial/ethnic groups in U.S.-based RA RCTs over the period 2008-2017, with significant underrepresentation of these groups throughout. The results also suggest that men are underrepresented in RA RCTs and that the elderly are often excluded from RA RCTs.
To cite this abstract in AMA style:
Strait A, Castillo F, Choden S, Li J, Whitaker E, Falasinnu T, Schmajuk G, Yazdany J. Clinical Trials in Rheumatoid Arthritis Have Inadequate Racial/Ethnic, Gender and Age Diversity: A Systematic Review [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/clinical-trials-in-rheumatoid-arthritis-have-inadequate-racial-ethnic-gender-and-age-diversity-a-systematic-review/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-trials-in-rheumatoid-arthritis-have-inadequate-racial-ethnic-gender-and-age-diversity-a-systematic-review/