Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Giant cell arteritis (GCA), the most common systemic vasculitis, may have diagnostic and treatment delays that can increase risk of vascular complications. Diagnostic color doppler ultrasound (US) has potential to decrease time to diagnosis, limit corticosteroid exposure, and avoid need for a surgical procedure, all at lower cost than temporal artery biopsy (TAB).
Identification of bottlenecks in patient workup and delays in treatment can guide targeted quality improvement interventions. Our goal was twofold: to determine institutional use of US and TAB and to construct a timeline of key clinical events for patients with suspected GCA.
Methods: All data was obtained from the integrated electronic health record at our community-based multi-site health system from a 4.5-year timeframe starting in 2015 with GCA by International Classification of Diseases-9 or 10 diagnostic codes, and TAB order dates were determined by current procedural terminology codes. Because our outcomes were agnostic to final diagnosis, we did not designate GCA classification criteria for all patients. Using retrospective chart review, we identified dates of milestone events for patients who underwent GCA US: earliest of common GCA symptoms from a predefined list, medical presentation, corticosteroid initiation, inflammatory marker evaluation, US, and TAB.
Where available, we calculated intervals by subtraction of event dates, and where unavailable, approximated based on clinical documentation. Mean durations were rounded to the nearest whole day and excluded negative integers (e.g. patient excluded if prescribed corticosteroids for different indication days prior to presentation with suspected GCA and new, high-dose corticosteroid indication).
Results: Comparing US and TAB among 274 patients with new GCA diagnoses, 100 (36%) had TAB only ordered, 29 (11%) had both TAB ordered and had US, and 12 (4%) had US only. Timeline values were as follows (mean ± standard deviation, in days): symptom onset to presentation 21 ± 43, presentation to corticosteroids 2 ± 3, presentation to labs 2 ± 5, presentation to US 12 ± 23, presentation to TAB 14 ± 14, corticosteroids to TAB 12 ± 9, corticosteroids to US 11 ± 27, and US to TAB 11 ± 9. Time from presentation to US versus TAB was not significantly different (p=0.77). Ten of 37 patients (27%) with calculable corticosteroid dates were not prescribed high-dose empiric corticosteroids within two days of presentation.
Conclusion: Patients with suspected GCA had widely variable times to key events in their clinical courses, including initial presentation. US was used in only a minority of suspected GCA cases. Future work could target interventions to reduce variability in diagnostic access and empiric therapy initiation, such as through a fast-track clinic model.
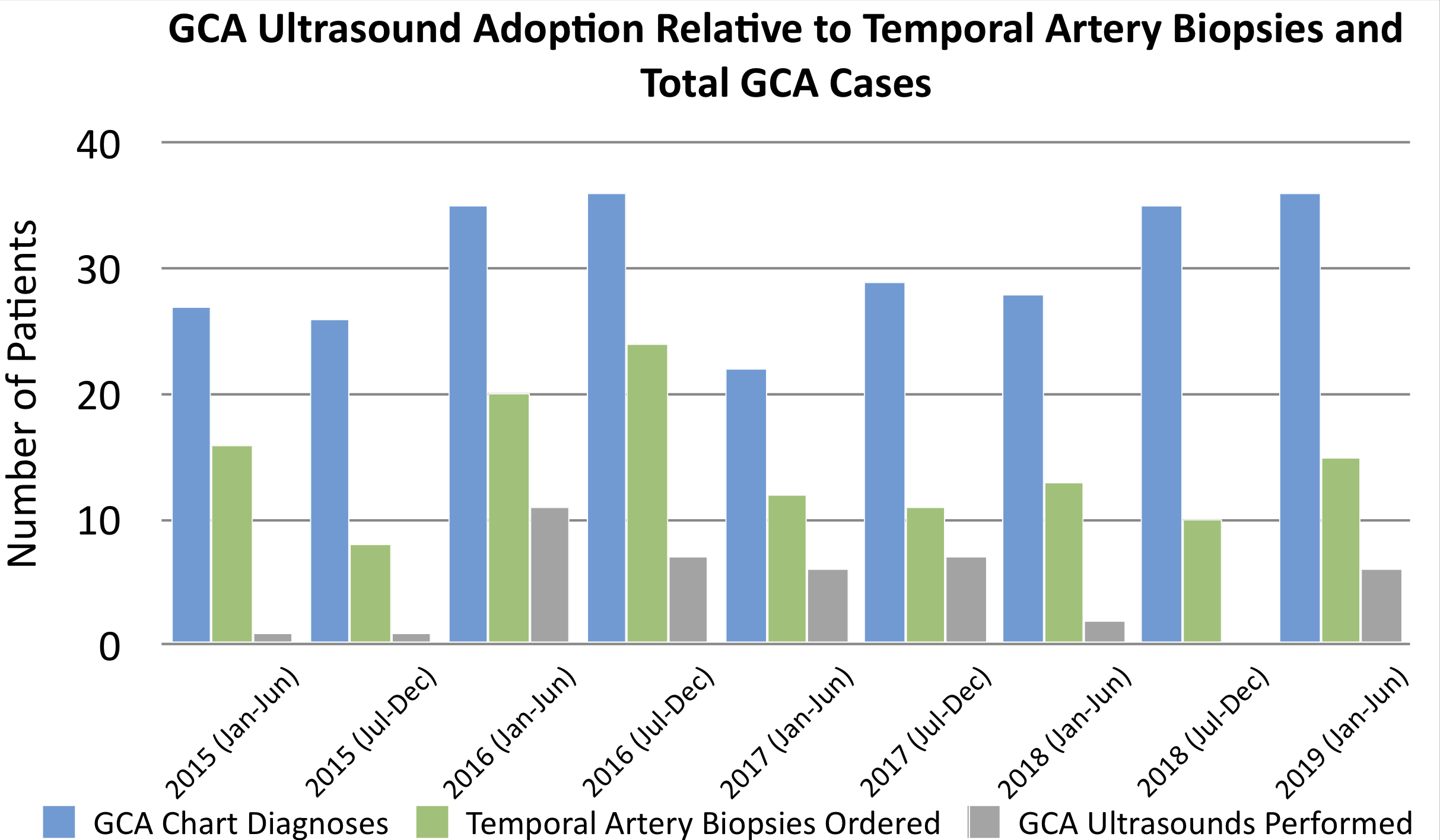 Comparing US and TAB among 274 patients with new GCA diagnoses, 100 (36%) had TAB only ordered, 29 (11%) had both TAB ordered and had US, and 12 (4%) had US only.
Comparing US and TAB among 274 patients with new GCA diagnoses, 100 (36%) had TAB only ordered, 29 (11%) had both TAB ordered and had US, and 12 (4%) had US only.
 Timeline mean intervals with standard deviation. Symptom onset to presentation 21 ± 43, presentation to corticosteroids 2 ± 3, presentation to labs 2 ± 5, presentation to US 12 ± 23, presentation to TAB 14 ± 14, corticosteroids to TAB 12 ± 9, corticosteroids to US 11 ± 27, and US to TAB 11 ± 9. Time from presentation to US versus TAB was not significantly different (p=0.77).
Timeline mean intervals with standard deviation. Symptom onset to presentation 21 ± 43, presentation to corticosteroids 2 ± 3, presentation to labs 2 ± 5, presentation to US 12 ± 23, presentation to TAB 14 ± 14, corticosteroids to TAB 12 ± 9, corticosteroids to US 11 ± 27, and US to TAB 11 ± 9. Time from presentation to US versus TAB was not significantly different (p=0.77).
To cite this abstract in AMA style:
Slade S, Chiu C, Bauer E, Dave A. Clinical Timelines and Management Delays in Suspected Giant Cell Arteritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/clinical-timelines-and-management-delays-in-suspected-giant-cell-arteritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-timelines-and-management-delays-in-suspected-giant-cell-arteritis/
