Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: While B cell signaling is thought to be important in the pathogenesis of systemic sclerosis (SSc), the experience with B cell targeting therapies in SSc clinical trials has been mixed. Belimumab is a monoclonal antibody that inhibits B cell survival. We investigated gene expression changes of skin biopsy specimens of patients with early diffuse cutaneous (dc) SSc treated in the context of a randomized controlled pilot trial of belimumab versus placebo in patients on background mycophenolate mofetil (MMF) therapy (NCT01670565).
Methods: Biopsies of lesional forearm skin were obtained from 18 patients prior to randomization and after 52 weeks of treatment with belimumab or placebo. Patients were defined as improvers if they had a modified Rodnan Skin Score improvement ≥20% post-treatment. Using this criterion, 7/9 patients in the belimumab/MMF group were improvers, and 3/9 in the placebo/MMF group were improvers. Expression data from skin biopsies were analyzed for differentially expressed genes (p≤0.05, paired/unpaired t-test) which were investigated for functional enrichment via g:Profiler (p≤0.05, corrected for multiple testing). Differentially expressed pathways were determined genome-wide using Gene Set Enrichment Analysis (False Discovery Rate ≤5%).
Results: In the belimumab/MMF group, clinical improvers showed downregulation of genes involved in B cell mediated immunity, extracellular matrix (ECM), collagen formation and response to TGF-β. Decreased pathways included IL4/IL6 signaling as well as B cell signaling, ECM, TGF-β and PDGF signaling (Figure 1). Comparison between baseline samples of improvers and non-improvers showed that patients who improved were characterized by the increased baseline expression of ECM and TGF-β signaling genes and pathways. In the improvers from placebo/MMF group, we observed decreased expression of genes and pathways involved in TCR signaling, e.g. T cell activation and T cell aggregation, consistent with the mechanism of MMF as well as Toll-like receptor signaling. In both treatment arms, non-improvers were characterized by a small number of differentially expressed genes with no functional enrichment, consistent with the lack of clinical response.
Conclusion: Decrease in B cell signaling genes and pathways was observed only in patients with improved skin score in belimumab/MMF but not placebo/MMF group. While attributable to the pharmacologic effect of the drug, this was not seen in patients who did not improve. Improvers from belimumab/MMF group showed high baseline expression and significant post-treatment decrease of fibrotic genes and pathways i.e. ECM, TGF-β and PDGF signaling. This finding suggests that treatment effect may be influencing the interplay between immune activation and fibrosis linked to clinical manifestations of SSc such as skin fibrosis. 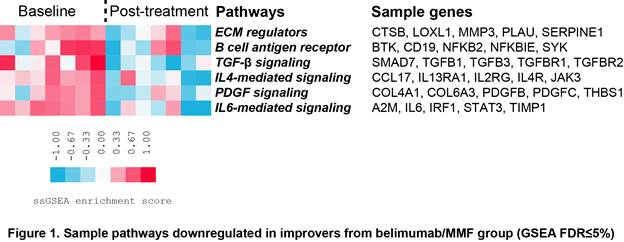
To cite this abstract in AMA style:
Martyanov V, Gordon JK, Wood TA, Spiera RF, Whitfield ML. Clinical Response to Treatment with Belimumab and Mycophenolate Mofetil Is Associated with Decrease in B Cell, TGF-β and PDGF Signaling in Systemic Sclerosis [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/clinical-response-to-treatment-with-belimumab-and-mycophenolate-mofetil-is-associated-with-decrease-in-b-cell-tgf-%ce%b2-and-pdgf-signaling-in-systemic-sclerosis/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-response-to-treatment-with-belimumab-and-mycophenolate-mofetil-is-associated-with-decrease-in-b-cell-tgf-%ce%b2-and-pdgf-signaling-in-systemic-sclerosis/
