Session Information
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: GiACTA, a randomized, double-blind, placebo (PBO)–controlled study, demonstrated the efficacy and safety of tocilizumab (TCZ) in patients with giant cell arteritis (GCA).1 Growing evidence has shown that TCZ is also effective for the treatment of polymyalgia rheumatica (PMR); however, data on this are limited.2,3 The purpose of this study was to evaluate the efficacy of TCZ in patients with GCA presenting with cranial symptoms only or PMR symptoms only in GiACTA.
Methods: GiACTA randomized 251 GCA patients to receive weekly or every other week TCZ plus a 26-week prednisone taper (TCZ + prednisone) or PBO plus a 26- or 52-week prednisone taper (PBO + prednisone).1 In this post hoc analysis, baseline characteristics, sustained remission rate, number of flares, annual flare rate, time to flare, cumulative prednisone dose and safety were assessed in patients with PMR symptoms only and patients with cranial symptoms only at diagnosis. Disease flare was defined as the recurrence of signs or symptoms of GCA (including PMR) or an elevation in erythrocyte sedimentation rate attributable to GCA.
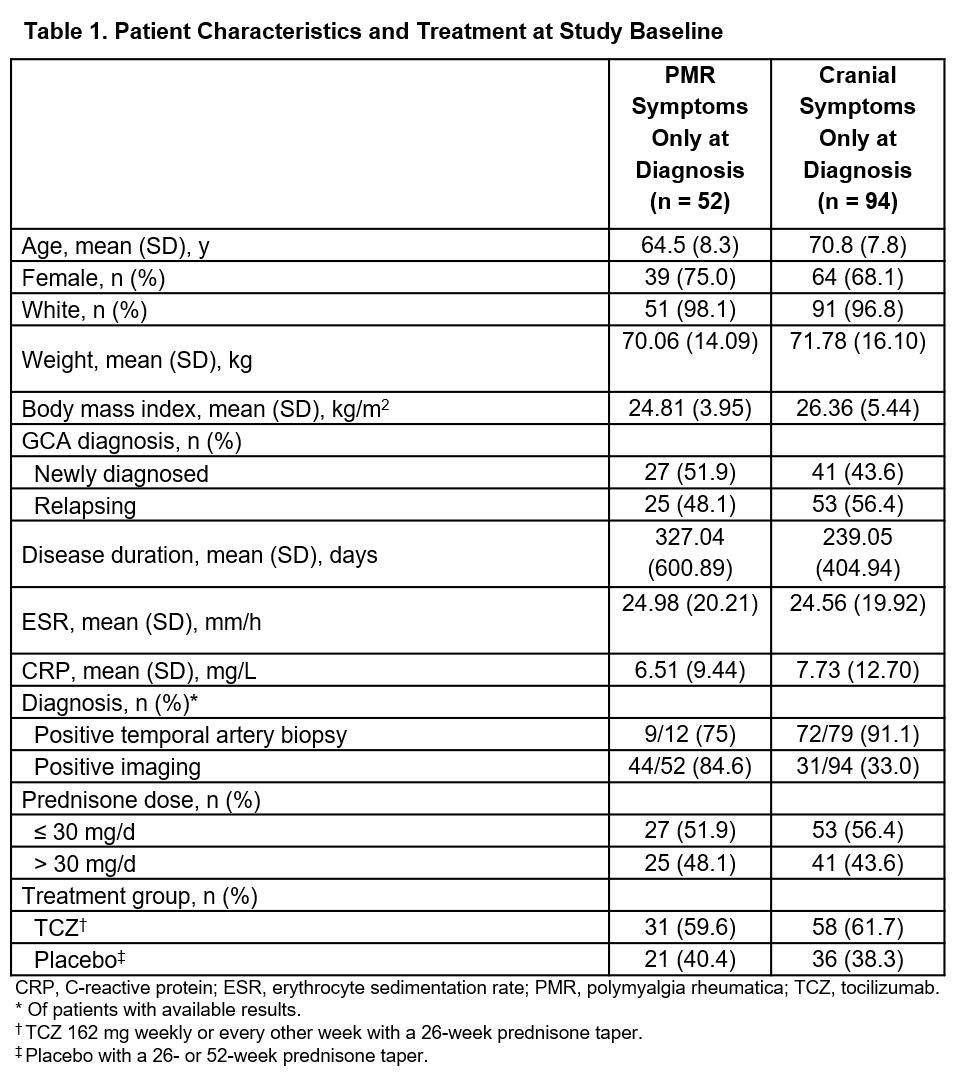
Results: Of 146 patients included in the analysis, 52 had PMR symptoms only and 94 had cranial symptoms only at diagnosis. Demographics and other patient characteristics are shown in Table 1. The hazard ratios for flare in patients receiving TCZ vs PBO were 0.77 (99% CI, 0.26-2.32) for patients with PMR symptoms only and 0.37 (99% CI, 0.13-1.01) for patients with cranial symptoms only. Of patients with PMR symptoms only, 18 flares occurred in 13/31 patients (41.9%) in the TCZ group and 20 flares occurred in 12/21 patients (57.1%) in the PBO group. Of patients with cranial symptoms only, 19 flares occurred in 12/58 patients (20.7%) in the TCZ group and 25 flares occurred in 17/36 patients (47.2%) in the PBO group (Table 2). For both the PMR and cranial symptoms only groups, annual flare rates were lower in patients receiving TCZ compared with those receiving PBO. The occurrence of adverse events and serious adverse events was similar between groups (Table 2).
Conclusion:
TCZ improved clinical outcomes in patients who presented with PMR symptoms only or cranial symptoms only at diagnosis as indicated by a reduced incidence of flares. These findings suggest that TCZ is effective in patients with GCA with PMR or cranial symptoms.
References:
- Stone JH, et al. N Engl J Med. 2017;377(4):317-328.
- Lally L, et al. Arthritis Rheumatol. 2016;68(10):2550-2554.
- Devauchelle-Pensec V, et al. Ann Rheum Dis. 2016;75(8):1506-1510.
Acknowledgements: This study was funded by Genentech, Inc. Support for third-party writing assistance, furnished by Health Interactions, Inc. was provided by Genentech, Inc.
To cite this abstract in AMA style:
Spiera R, Unizony S, Bao M, Luder Y, Sidiropoulos P, Han J, Pei J, Stone J. Clinical Outcomes of Patients with Giant Cell Arteritis with Polymyalgia Symptoms Only vs Cranial Symptoms Only Treated with Tocilizumab or Placebo in a Randomized Clinical Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/clinical-outcomes-of-patients-with-giant-cell-arteritis-with-polymyalgia-symptoms-only-vs-cranial-symptoms-only-treated-with-tocilizumab-or-placebo-in-a-randomized-clinical-trial/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-outcomes-of-patients-with-giant-cell-arteritis-with-polymyalgia-symptoms-only-vs-cranial-symptoms-only-treated-with-tocilizumab-or-placebo-in-a-randomized-clinical-trial/