Session Information
Date: Tuesday, October 23, 2018
Title: Vasculitis Poster III: Immunosuppressive Therapy in Giant Cell Arteritis and Polymyalgia Rheumatica
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Previously, the GiACTA study demonstrated the superiority of subcutaneous (SC) tocilizumab (TCZ) plus prednisone vs prednisone alone in achieving sustained glucocorticoid (GC)-free remission in patients with giant cell arteritis (GCA)1. We aimed to evaluate the effectiveness and safety of SC and intravenous TCZ in real-world clinical practice.
Methods: We performed a retrospective analysis of GCA patients treated with TCZ at a single center (MGH) between 2010-2018. Time to disease relapse, number of relapses, prednisone use, and adverse events (AE) before and after TCZ initiation were assessed. Disease relapse was defined as the re-appearance of clinical manifestations of GCA (e.g., cranial symptoms) that required treatment modification.
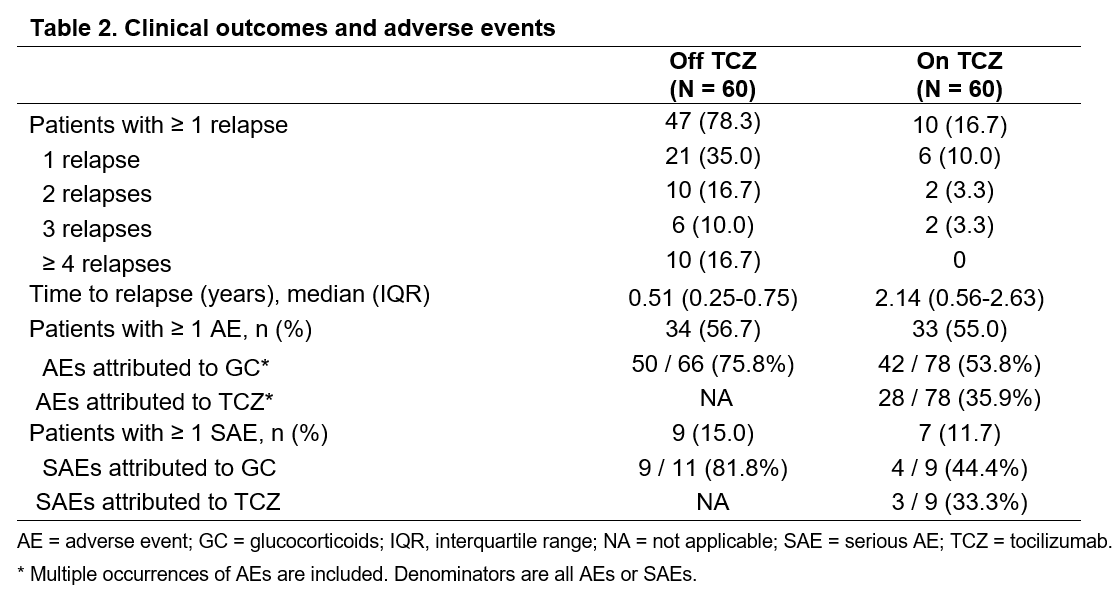
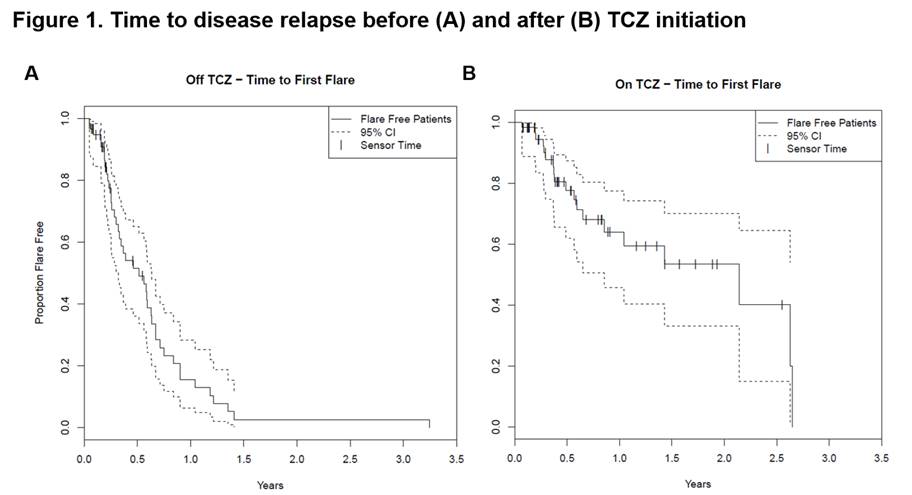
Results: A total of 60 GCA patients were included in the analysis. Table 1 depicts the baseline characteristics and the treatments received by this cohort. The median (IQR) disease duration before TCZ use was 0.6 (0.2-1.6) years. Fifty-eight patients (96.7%) received concomitant prednisone (mean [SD] dose: 30 [18.3] mg daily) at the time of TCZ initiation. Patients received TCZ for a median (IQR) period of 0.5 (0.3-1.4) years. While not on TCZ treatment, 47 patients (78.3%) had ≥1 relapse (median [IQR] time to flare 0.5 [0.3-0.8] years). On TCZ, 10 patients (16.7%) had ≥1 relapse (median [IQR] time to flare 2.1 [0.6-2.3] years) (Table 2, Figure 1). Twenty-four patients (41.4%) successfully tapered off prednisone during TCZ treatment. The incidence of AEs and serious AEs (SAE) was similar before and after TCZ initiation (Table 2). During TCZ treatment, however, 42 out of 78 AEs were considered related or possibly related to prednisone. An AE led to TCZ discontinuation in 5 patients. No deaths occurred during the study period.
Conclusion: In this retrospective analysis, TCZ improved clinical outcomes in patients with GCA as indicated by a reduced incidence of relapses and by the ability to discontinue prednisone. The occurrence of AEs and SAEs (many due to GC) did not differ substantially while patients were on or off TCZ. These real-world findings support the previously reported efficacy and safety profile of TCZ in patients with GCA.
References: 1. Stone JH, et al. N Engl J Med. 2017;377(4):317-328.
To cite this abstract in AMA style:
Unizony SH, Pei J, Sidiropoulos PN, Best JH, Birchwood C, Stone JH. Clinical Outcomes of Patients with Giant Cell Arteritis Treated with Tocilizumab in Real-World Clinical Practice [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/clinical-outcomes-of-patients-with-giant-cell-arteritis-treated-with-tocilizumab-in-real-world-clinical-practice/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-outcomes-of-patients-with-giant-cell-arteritis-treated-with-tocilizumab-in-real-world-clinical-practice/