Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Patients with CTD-ILD are often treated with a combination of corticosteroids and steroid sparing agents to limit the progression of the inflammatory response in the lung and prevent remodeling that can lead to fibrosis. While tapering regimens for steroids are part of the standard of care in CTD-ILD, there is no significant experience on outcomes in patients who taper steroid sparing agents for ILD. In this study, we identified those patients with CTD-ILD who were stable from a pulmonary perspective and who then tapered or discontinued their steroid sparing therapy in conjunction with their treating physician. By comparing physiologic, radiographic, and functional parameters before and after taper, we assessed for stability, improvement, or worsening of their ILD.
Methods: We retrospectively identified 22 patients with any type of CTD-ILD who attended a tertiary care multidisciplinary ILD clinic and had been treated with immunosuppressive agents, including mycophenolate, azathioprine or any agent that was intended to be used as a steroid sparing agent for CTD- ILD. Based on medical records, we reviewed changes in clinical status (dyspnea/cough as assessed by the treating clinician), serial pulmonary function testing and radiographic changes by CT per clinical radiology report. Assessment was made at 3 time points: initial presentation to ILD clinic, at initiation of medication taper, and after the medication had been tapered to its lowest point. Change of FVC >10% or DLCO >15% on PFT was considered significant.
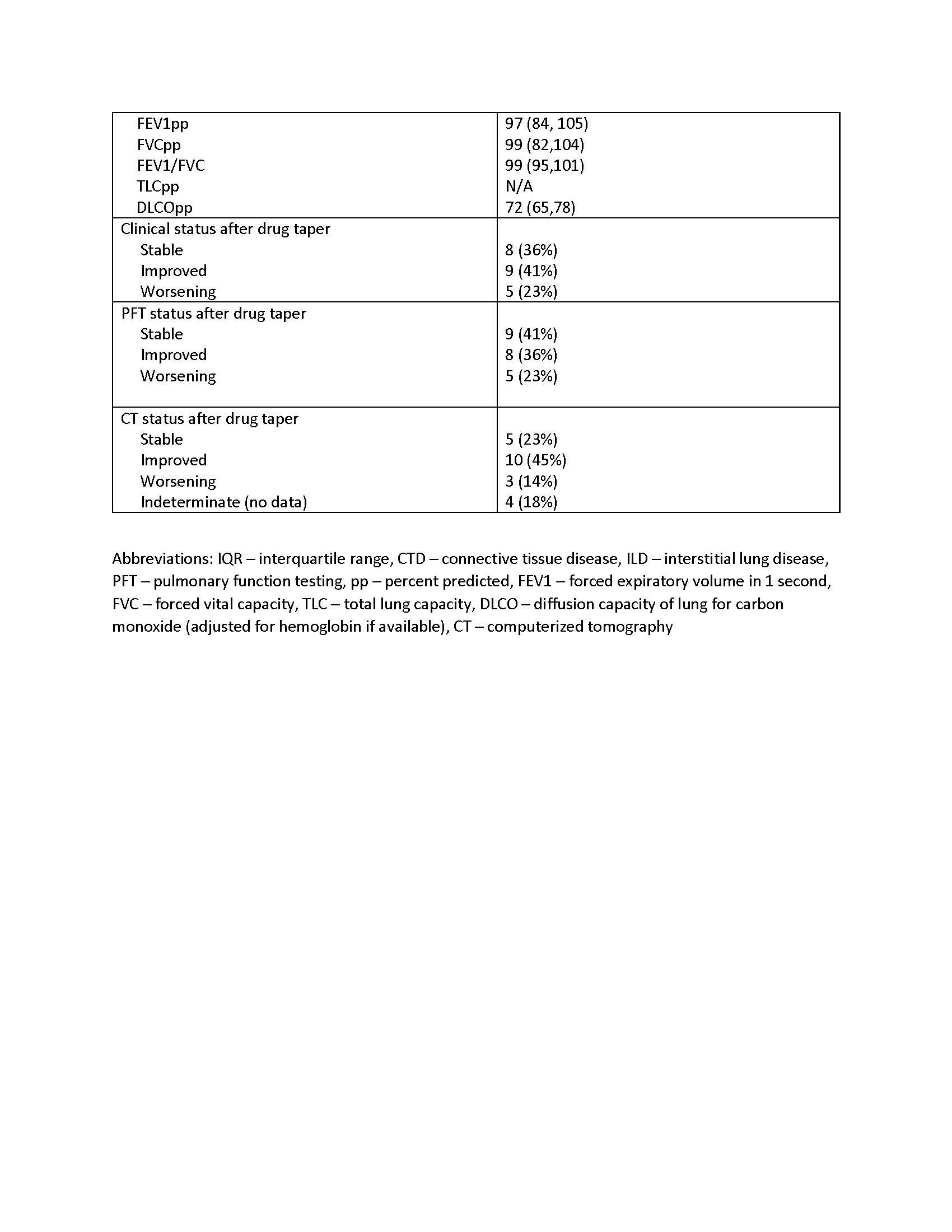
Results: The majority of patients had idiopathic pneumonia with autoimmune features (IPAF, n=13), followed by systemic sclerosis (n=4), myositis/antisynthetase syndrome (n=4), and systemic lupus erythematosus/Sjogren’s (n=1). Most patients requested to taper their medication based on a period of stability or with resolution of their ILD, understanding the risk of flare of their disease. Two patients requested taper due to concerns for immunosuppression and risk of infection and one patients’ mycophenolate was lowered due to liver function test abnormalities. Only one patient tapered off completely. The mean period of therapy before initiating taper was 22 months. 15/22 patients (68%) were stable or improved on tapered therapy whereas 7 (32%) patients declined in either clinical status, PFTs or CT. Five patients (23%) reported a clinical decline–of these 3/5 had worsening on CT and 3/5 had a decline in PFTs. Of the 3 with worsening on CT, one developed significant fibrotic progression despite reinstating therapy, one had mild progression in fibrosis and the other regained prior lung function and improvement on CT with reinstitution of therapy. Two persons had a decline in PFT but no change clinically or by CT.
Conclusion: Amongst 22 patients with clinically stable CTD-ILD who underwent taper of their steroid sparing medication, 32% had a decline in at least one of the 3 parameters used to assess lung function. Given the limits of this small observational cohort, a prospective study would be necessary to assess the safety of tapering steroid sparing therapy in patients with stable CTD-ILD.
To cite this abstract in AMA style:
Dellaripa P, Hoover P, Doyle T. Clinical Outcomes in an Observational Retrospective Cohort of Patients with Connective Tissue Disease-associated Interstitial Lung Disease (CTD-ILD) Who Taper Immunosuppressive Therapy [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/clinical-outcomes-in-an-observational-retrospective-cohort-of-patients-with-connective-tissue-disease-associated-interstitial-lung-disease-ctd-ild-who-taper-immunosuppressive-therapy/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-outcomes-in-an-observational-retrospective-cohort-of-patients-with-connective-tissue-disease-associated-interstitial-lung-disease-ctd-ild-who-taper-immunosuppressive-therapy/