Session Information
Date: Sunday, October 21, 2018
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Checkpoint immunotherapy has become the standard of care in treating advanced melanoma. These medications have been associated with immune-related adverse events (irAEs). Accurate methods of predicting and managing irAEs are still lacking. Here, we aim to identify clinical correlates of irAEs from patients with melanoma previously treated with immunotherapy.
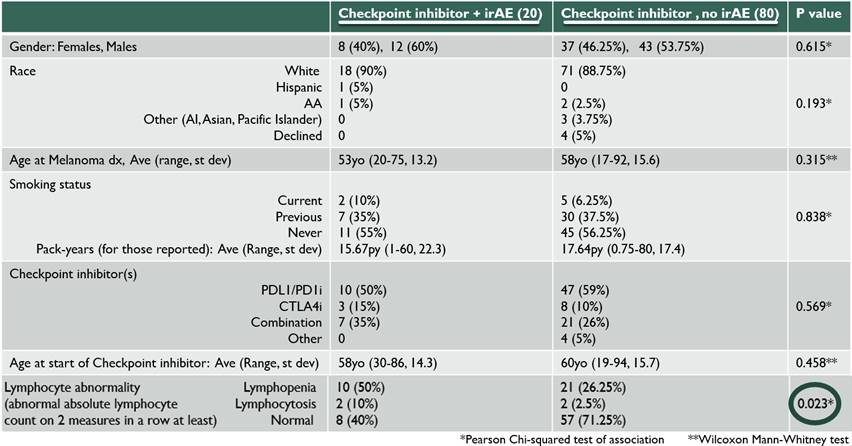
Methods: We retrospectively reviewed electronic medical records of patients with melanoma who were seen at The University of Chicago Medical Center between 1/2011-1/2017 and were treated with immune checkpoint inhibitors (ICIs). Other inclusion criteria included the following: ≥18 years old, ICI administered at least once, received systemic corticosteroids (CS). Patients’ irAEs were graded based on the National Cancer Institute’s Common Terminology Criteria for Adverse Events. Patients were excluded if they had low grade (1 or 2) irAEs and if systemic CS were used for reasons other than irAE management. Wilcoxon Mann-Whitney test and Chi-squared test of association were used to compare quantitative and categorical data (respectively) between patients with irAEs and patients without irAEs.
Results: Twenty patients with grade 3 or higher irAEs were identified and compared with 80 patients who received ICI therapy but did not have any irAEs. The irAEs were as follows: 4 transaminitis, 4 dermatitis, 3 arthritis, 3 colitis, 2 neurologic, 2 thyroiditis, 1 myocarditis, and 1 pneumonitis. Some patients had more than one high grade irAE with second irAE including iritis, Guillain-Barre syndrome, pancreatitis and adrenal insufficiency (AI). From start of ICI, the average days to first irAE occurrence was 121 days (7-378 days). The average days to irAE after cessation of ICI was 61 days (0-375 days), excluding an outlier of a late AI at 1316 days. There was no statistically significant difference between irAE and non-irAE patients in terms of gender, race, age at melanoma diagnosis, age at ICI therapy, or type of ICI used (p values 0.615, 0.193, 0.315, 0.458 and 0.569, respectively). There was a statistically significant difference in peripheral absolute lymphocyte count (ALC) between the two groups; while 60% of patients with irAEs had ALC abnormalities (lymphopenia defined as ALC<900/uL, lymphocytosis>3300/uL), only 28.7% of the group without irAEs had any peripheral ALC abnormalities. The 3 irAE patients with eosinophilia (>600/uL) had dermatitis.
Conclusion: This cohort evaluated patients with Melanoma treated with ICIs and found no significant demographic differences between those who developed a high grade irAE compared with those who did not. There was a significant difference in ALC, with those patients who developed irAEs demonstrating an abnormal ALC. More research is needed to further validate and elucidate this observed association between peripheral lymphocyte count and development of irAEs.
To cite this abstract in AMA style:
Reid P, Chongsuwat T, Dua A, Luke J. Clinical Correlates of Immune-Related Adverse Events for Patients with Melanoma Treated with Checkpoint Inhibitors and a Noted Significant Difference in Peripheral Lymphocyte Counts [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/clinical-correlates-of-immune-related-adverse-events-for-patients-with-melanoma-treated-with-checkpoint-inhibitors-and-a-noted-significant-difference-in-peripheral-lymphocyte-counts/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-correlates-of-immune-related-adverse-events-for-patients-with-melanoma-treated-with-checkpoint-inhibitors-and-a-noted-significant-difference-in-peripheral-lymphocyte-counts/