Session Information
Date: Monday, November 8, 2021
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: A recent study reports that gut inflammation is linked to degree of bone marrow edema in sacroiliac joints in patients with axial spondyloarthritis (SpA). SpA patients with concomitant inflammatory bowel disease (IBD) typically have more severe disease requiring aggressive treatment. However, the clinical characteristics of SpA patients with IBD has not been shown in a large cohort and there are few descriptions of regional differences.
The purpose of this study is to clarify the clinical characteristics of SpA patients with IBD compared to those SpA patients without IBD. In addition, we aim to determine the phenotype of patients given a definitive diagnosis of IBD-associated SpA by their treating rheumatologist.
Methods: Using ASAS-PerSpA data, an observational study, we analyzed information on demographics and disease characteristics, dichotomizing patients by IBD status. SpA patients with IBD were further categorized by region; Japan, non-Japan Asia, and non-Asian countries. SpA patients with IBD were also categorized as IBD-associated SpA or other SpA with IBD by their rheumatologists.
Results: Among 4465 SpA patients included in the study, 287 were identified with IBD. Compared to those without IBD, SpA patients with IBD were less likely male (54.0 vs 61.5%) and more likely to have experienced diagnostic delay (5.1 vs 2.9 years) (Table 1). SpA patients with IBD had lower prevalence of positive HLA-B27 (37.2% vs 68.0%) and less dactylitis (9.1% vs 15.8%) despite similar rates of other peripheral signs. Disease activity, radiographic sacroiliitis and inflammation on MRI were similar in the two groups. csDMARDs and bDMARDs use was higher in SpA patients with IBD.
With respect to regional differences, SpA patients with IBD was more common in Japan than in both non-Japan Asia and non-Asian countries (13.2, 1.7 and 7.1%, respectively). SpA patients with IBD in Japan experienced more diagnostic delay (12.8, 0.3 and 5.0 years, respectively), had lower prevalence of positive HLA-B27 (0, 81.8 and 36.2%, respectively) and less axial symptom (inflammatory back pain: 57.7, 84.6 and 81.9%, radiographic sacroiliitis: 23.1,46.2 and 60.9%, sacroiliitis on MRI: 33.3, 83.3 and 71.3%, respectively).
SpA patients with IBD were categorized by rheumatologists into their respective clinical diagnoses, of which 111 were diagnosed with IBD-associated SpA (Figure 1). IBD-associated SpA patients, compared to those diagnosed as primarily SpA patients with IBD but not IBD-associated SpA, had lower prevalence of both HLA-B27 (19.3 vs 45.2%) and family history of SpA (24.3 vs 39.8%) (Table 2). IBD-associated SpA patients had fewer axial symptom and signs, and were more likely to have peripheral arthritis – especially oligoarthritis. In IBD-associated SpA patients, IBD appears more often as the first symptom of SpA, and IBD-specific treatment was needed more frequently. csDMARDs use was higher in IBD-associated SpA.
Conclusion: SpA patients with IBD required more specific treatments than those without IBD. In SpA patients with IBD, rheumatologists tended to diagnose IBD-associated SpA in those without axial signs but with peripheral signs, and those with IBD as the initial symptom and requiring IBD-specific treatment.
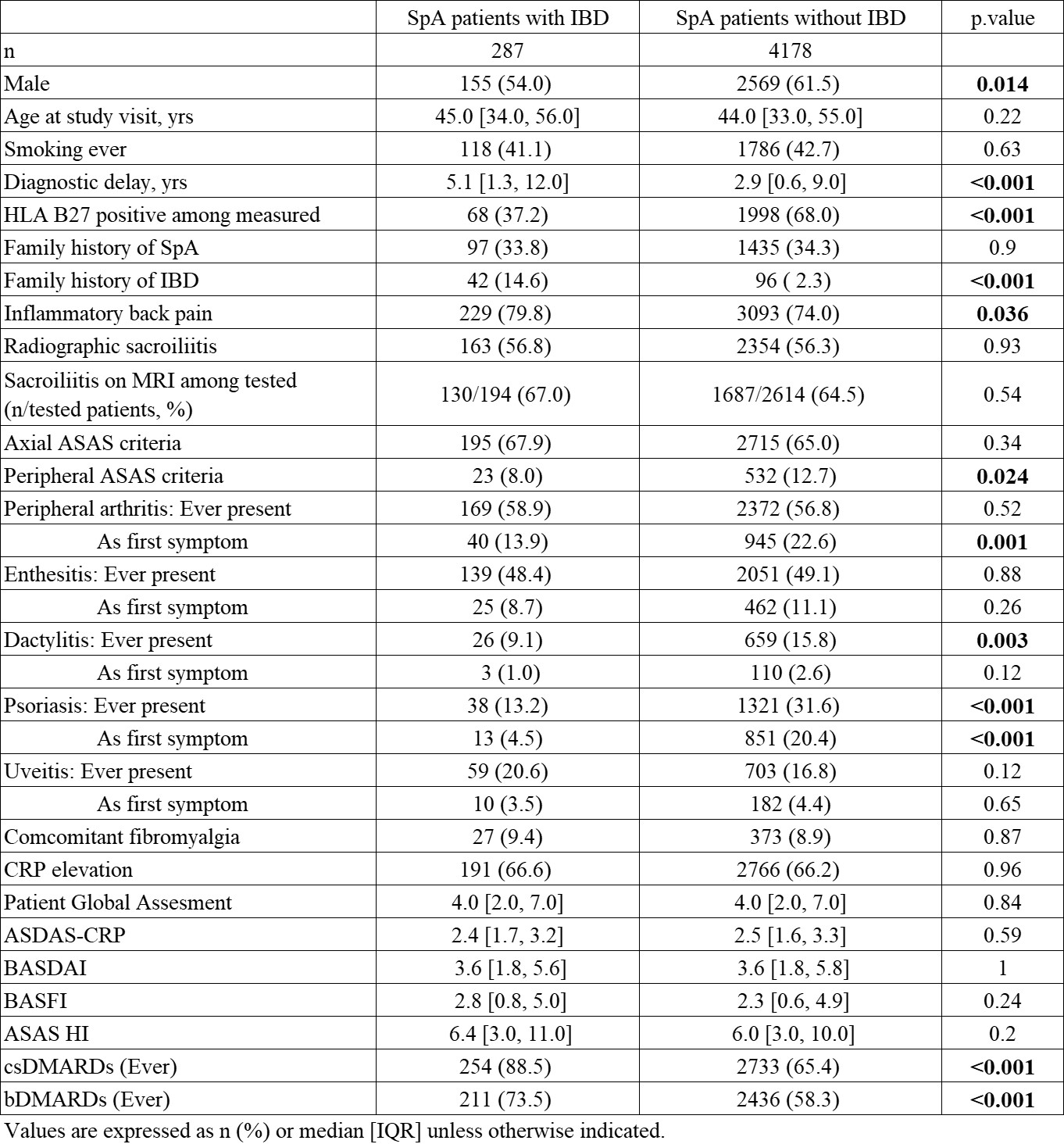 Table 1. Clinical Characteristics of SpA patients with IBD.
Table 1. Clinical Characteristics of SpA patients with IBD.
 Figure 1. Final clinical diagnosis of SpA patients with IBD patients by rheumatologists. (Nf287)
Figure 1. Final clinical diagnosis of SpA patients with IBD patients by rheumatologists. (Nf287)
 Table 2. Clinical Characteristics of IBD-associated SpA compared to other SpA patients with IBD.
Table 2. Clinical Characteristics of IBD-associated SpA compared to other SpA patients with IBD.
To cite this abstract in AMA style:
Ono K, Kishimoto M, Deshpande G, Fukui S, Kawaai S, Sawada H, Matsuura M, Rios Rodriguez V, Proft F, Tada K, Tamura N, Taniguchi Y, Hirata A, Kameda H, Tsuji S, Kaneko Y, Dobashi H, Okano T, Haji Y, Morita A, Asahina A, Okada M, Komagata Y, López Medina C, Molto A, van der Heijde D, Dougados M, Hisamatsu T, Tomita T, Kaname S. Clinical Characteristics of Patients with SpA and Concomitant IBD: Results from the ASAS PerSpA Study [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/clinical-characteristics-of-patients-with-spa-and-concomitant-ibd-results-from-the-asas-perspa-study/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-characteristics-of-patients-with-spa-and-concomitant-ibd-results-from-the-asas-perspa-study/
