Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The SRI (SLE responder index) is a composite measure established as a primary endpoint in SLE clinical trials. However, it has been questioned whether the SRI response represents a clinically meaningful change in disease state. Therefore, in order to clarify the clinical relevance of achieving the SRI, we studied clinical and laboratory correlates of the response.
Methods: Patients with SELENA-SLEDAI (SS) score ≥8, receiving stable standard of SLE care (SoC) for ≥30 days were randomized (2:1) to weekly belimumab (BEL) 200 mg or placebo (PBO), subcutaneously (prefilled syringe), plus SoC in the BLISS-SC clinical trial (BEL112341; NCT01484496). The primary endpoint of the trial was the SRI response at week 52 (≥4-point SS reduction, no worsening [<0.3 increase] in Physician’s Global Assessment, and 0 new BILAG A or ≤1 new BILAG B organ domain scores, all vs baseline). Regardless of treatment received, SRI responders were compared with SRI non-responders based upon changes in clinical and laboratory parameters assessed at week 52 using the Fisher’s exact test, logistic regression, analysis of covariance, and Cox regression, as appropriate.
Results: Of the 833 patients who reached week 52, 475 were SRI responders and 358 were SRI non-responders (observed population). SRI responders had statistically significantly better outcomes in multiple domains: improvements in the individual SRI components, number of improved SS and BILAG organ domain systems, reduction in prednisone use, less SFI flares (overall and severe), improvement in the FACIT-Fatigue score, and improvements in complement and anti-dsDNA antibody levels. CD20+ B cells were similar between the two groups. These results are summarized in Table 1. Similar observations have been seen in the Phase 3 BLISS trials following intravenous administration of belimumab or placebo plus SoC (1). 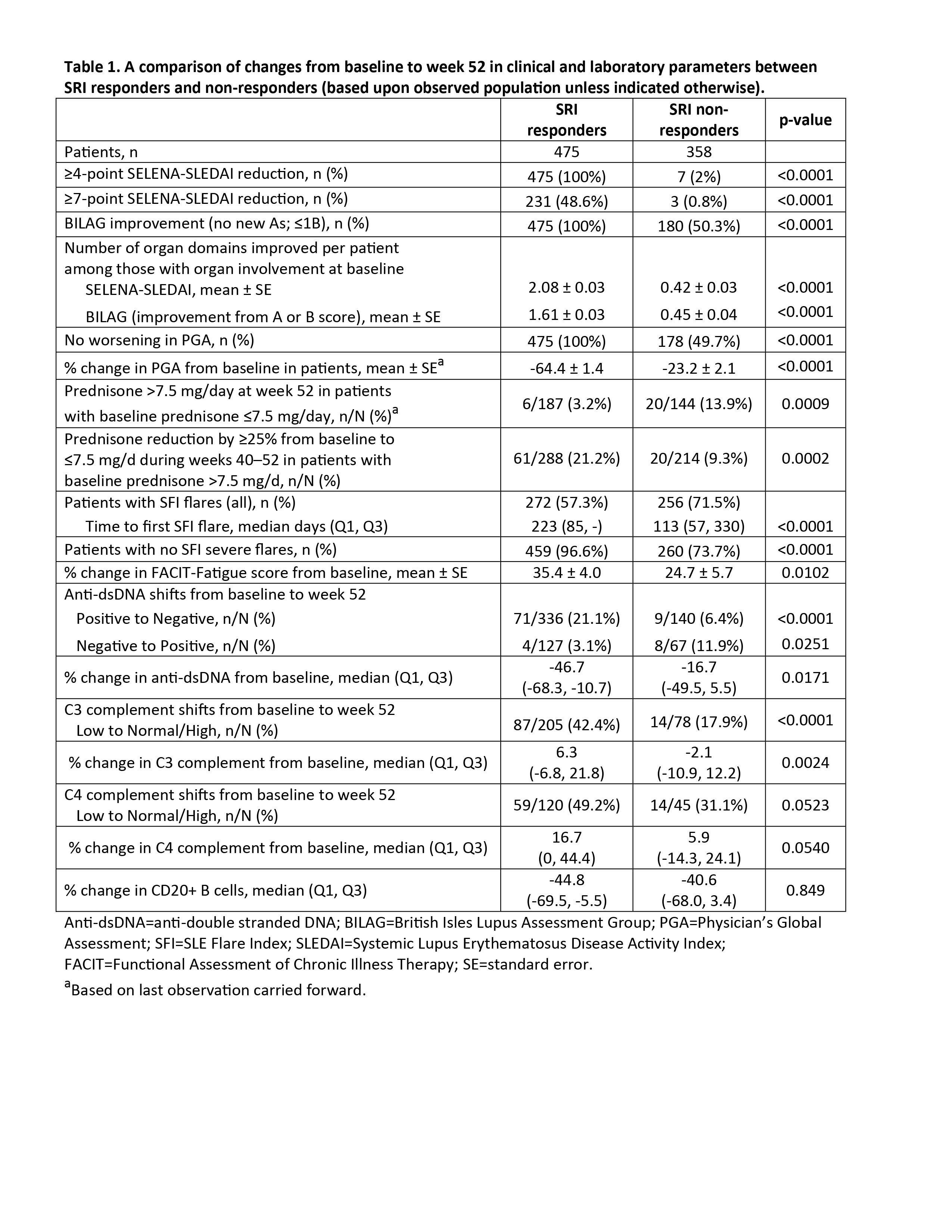
Conclusion: Patients who were SRI responders in this trial, regardless of treatment, had numerous clinical and serological benefits versus non-responders, providing strong support that the SRI response represents a clinically meaningful outcome for patients with SLE. Reference
1. Furie R et al. Lupus Sci Med 2014;1:e000031.doi:10.1136/lupus-2014-000031. Disclosures: GlaxoSmithKline/Human Genome Sciences sponsored and conducted this clinical trial.
To cite this abstract in AMA style:
van Vollenhoven RF, Stohl W, Furie R, Fox NL, Groark J, Bass D, Kurtinecz M, Pobiner B, Eastman W, Gonzalez-Rivera T, Gordon D. Clinical and Laboratory Correlates of Response in a Phase 3 Clinical Trial of Belimumab or Placebo Administered Subcutaneously Plus Standard Care to Patients with Systemic Lupus Erythematosus (SLE) [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/clinical-and-laboratory-correlates-of-response-in-a-phase-3-clinical-trial-of-belimumab-or-placebo-administered-subcutaneously-plus-standard-care-to-patients-with-systemic-lupus-erythematosus-sle/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-and-laboratory-correlates-of-response-in-a-phase-3-clinical-trial-of-belimumab-or-placebo-administered-subcutaneously-plus-standard-care-to-patients-with-systemic-lupus-erythematosus-sle/
