Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose
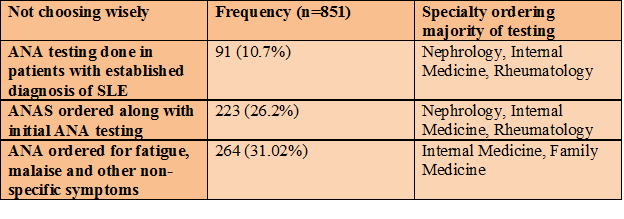
Choosing Wisely campaign aims to promote evidence based care that is truly necessary and not duplicative. ACR has furnished guidelines for use of ANA and ANA subserologies (ANAS)1: First, repeat testing of ANA is not indicated in patients with established diagnosis of SLE. Second, ANAS should not be ordered along with initial ANA testing. Third, ANA should not be used as a screening test in patients with nonspecific symptoms. The study objective was to identify occasions where ANA testing does not reflect high-value, cost-conscious care.
Methods
All patients having ANA test done at St Peter’s Hospital lab from 4/1/2012 to 3/31/2014 were included. Retrospective chart review was done for demographics, indication of testing and outcomes. Inferential analysis and logistic regression were done by SAS 9.0.
Results
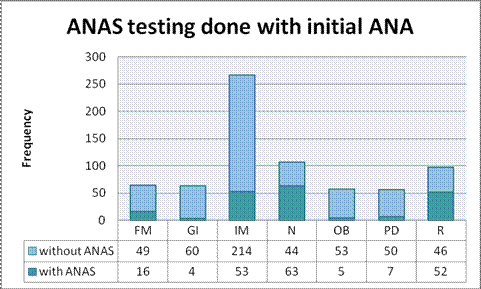
Total 851 patient encounters were found with mean age of 46.9 years and M:F ratio of 1:3.3. In 91 encounters, ANA testing was done despite a history of SLE. In 223 encounters, ANAS were ordered along with initial ANA. Of 223 ANAS ordered, only 15 were positive; all of which had positive ANA results. Female patients (OR 2.92, 95% CI 1.86-4.59) and those with history of autoimmune disease (AID) (OR 6.76, 95% CI 4.37-10.44) were more likely to have a positive ANA result. Patients with history of AID (OR 7.87, 95% CI 1.72-35.95) and those with a positive ANA results (p<0.001) were more likely to have positive ANAS. Age (OR 0.99, 95% CI 0.98-1.01) and location of patient (p=0.48) did not show association with ANA results. ANA testing ordered in family medicine, pediatrics and gastroenterology were more likely to have negative results (OR 2.61, 2.3 and 5.04 respectively) as compared to rheumatology. Follow up visits were available in 587 encounters; on 12 occasions (2.07%) a positive ANA result translated in a change in management.
Conclusion
A significant amount of clinically done ANA testing does not reflect high value care and is associated with significant economic burden (>150,000 USD/yr).
Reference
1. Yazdany J et al. Arthritis care & research 2013;65:329-39.
Table 1: ANA testing behavior of different specialties in patients with history of SLE.
Table 2: ANAS testing behavior of different specialties along with initial ANA testing.
Table 3: Clinical situations where ANA testing does not reflect high-value care
Disclosure:
T. Sheth,
None;
D. Alcid,
None.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/choosing-not-so-wisely-the-tale-of-antinuclear-antibody-testing/