Session Information
Date: Monday, November 8, 2021
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster III: Outcomes (1257–1303)
Session Type: Poster Session C
Session Time: 8:30AM-10:30AM
Background/Purpose: SLE is a chronic, autoimmune disease affecting multiple organ systems, and characterized by fluctuating disease activity. Many SLE disease measures may be impractical for routine clinical practice due to administrative burden and delay in scoring while awaiting laboratory results. Patients may also feel discouraged if their laboratory results indicate improvement while their physical/mental well-being has not improved. Rheumatoid arthritis (RA)–specific disease measures are widely used to assess patients with RA. This study evaluated the use of RA disease measures among patients with SLE in United Rheumatology’s community-based physician network in the United States.
Methods: A retrospective, observational analysis (GSK Study 213818) was conducted using the United Rheumatology Normalized Integrated Community Evidence (UR NICE) electronic medical records database. Patients ≥5 years of age diagnosed with SLE (≥1 encounter with an SLE International Classification of Disease-9 or 10 code) between January 1, 2010 and December 31, 2014 were assessed (date of first SLE diagnosis = index date). At least 5 years of clinical activity post-index was required. Patient characteristics were reported at index date, and certain RA disease measures were assessed during a 5-year follow-up period (Patient/Physician Global Assessment [PtGA/PGA]; Pain Index; tender/swollen joint counts [TJC/SJC]; Health Assessment Questionnaire [HAQ]; Routine Assessment of Patient Index Data 3 [RAPID3]), as counts and percentages, or means and standard deviations.
Results: Overall, 5990 patients with SLE and 5 years’ follow-up met the inclusion criteria (20–59 years of age, 73.8%; female, 91.8%; commercial insurance, 63.7%; comorbid RA post-index, 9–11%) (Table 1). The most frequently used RA assessments included: Pain Index; TJC/SJC; HAQ, PtGA; PGA; and RAPID3. In Year 1, use of RA measures ranged from 2.0% for PGA to 36.1% for Pain Index; use increased in Year 5 (ranging from 20.0% for PGA to 59.2% for Pain Index). Across the 5-year follow-up period, mean PtGA scores were consistently higher (indicating greater disease activity) than PGA scores. Mean scores for Pain Index and TJC/SJC were relatively stable throughout the 5-year period (Pain Index, 4.1 to 4.2; SJC, 0.9 to 1.1; TJC, 3.2 to 3.5). Similar findings were observed for HAQ and RAPID3 (HAQ: 1.5 to 2.0; RAPID3: 3.6 to 4.3). However, the proportion of patients classified as in remission or low disease activity by RAPID3 increased over the 5-year period (Year 1, 18.0%; Year 5, 27.9%) (Table 2).
Conclusion: RA disease measures were used, albeit infrequently, to assess patients with SLE in a rheumatology physician network. PtGA scores consistently indicated greater disease activity than the PGA scores, which may correspond to the discordance of Pain Index and TJC/SJC results over the 5-year period. Further research is needed to better understand the utility of various RA assessments for evaluating patients with SLE. However, they may ultimately represent an efficient approach to measuring patient outcomes and improving care.
Funding: GSK
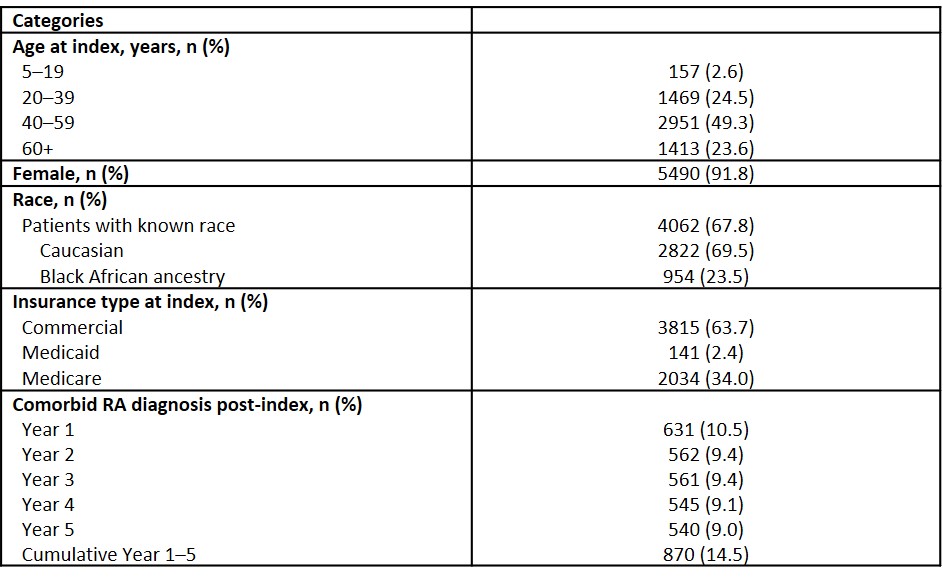 Table 1. Patient characteristics (Nf5990)
Table 1. Patient characteristics (Nf5990)
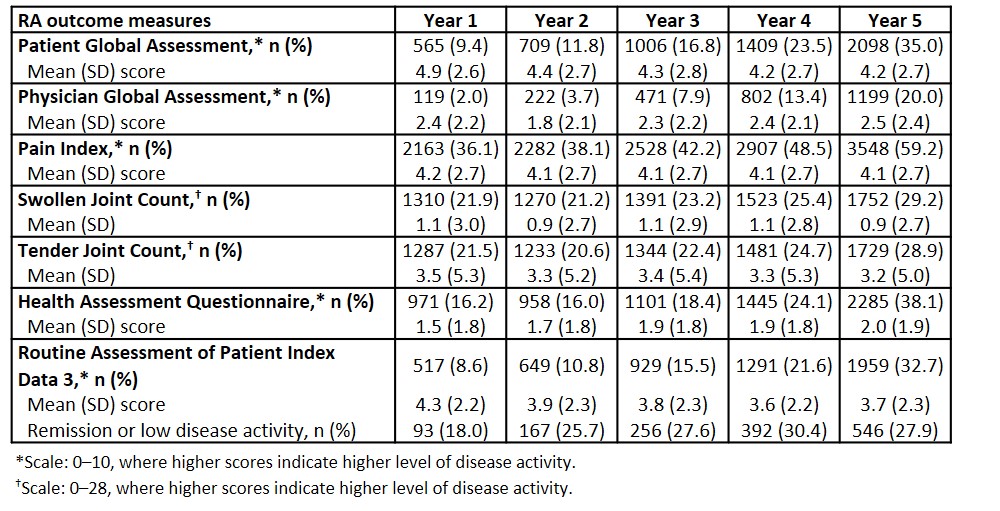 Table 2. RA outcome measures for patients with SLE during the 5-year follow-up period (Nf5990)
Table 2. RA outcome measures for patients with SLE during the 5-year follow-up period (Nf5990)
To cite this abstract in AMA style:
Bell C, Huang S, Wang M, DerSarkissian M, Duh M, Dhillon B, Averell C, Rubin B, Wallace D. Characterizing Patient and Physician Perceptions of Systemic Lupus Erythematosus (SLE) Disease Burden Using Traditional Rheumatoid Arthritis Outcomes Measures [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/characterizing-patient-and-physician-perceptions-of-systemic-lupus-erythematosus-sle-disease-burden-using-traditional-rheumatoid-arthritis-outcomes-measures/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/characterizing-patient-and-physician-perceptions-of-systemic-lupus-erythematosus-sle-disease-burden-using-traditional-rheumatoid-arthritis-outcomes-measures/
