Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: FDA-approved for moderate-severe SLE in August 2021, anifrolumab is a type I interferon (IFN) receptor antagonist that has been shown to decrease lupus flares and reduce corticosteroid use. Here we provide real world data describing its use in daily rheumatology practice, focusing on the clinical/laboratory characteristics and previous treatments of SLE patients who were prescribed anifrolumab for the first time.
Methods: Data were collected from patients under the care of ARN providers using PIONEER Rheumatology, an enhanced database combining fielded EMR data with extracted information from open text (office visits, infusion logs, and provider-patient communications). The study population consisted of adult (18+ years old) patients with documented diagnosis of SLE at least twice who were prescribed anifrolumab between August 1, 2021, to March 30, 2023. The index date for each patient was defined as the initial prescription date and baseline measures were those occurring within 365 days prior to the index date. Reasons for prior therapy discontinuation were extracted from office visit notes by clinically-trained scribes and categorized as lack/loss of efficacy, clinical reasons, or non-clinical reasons.
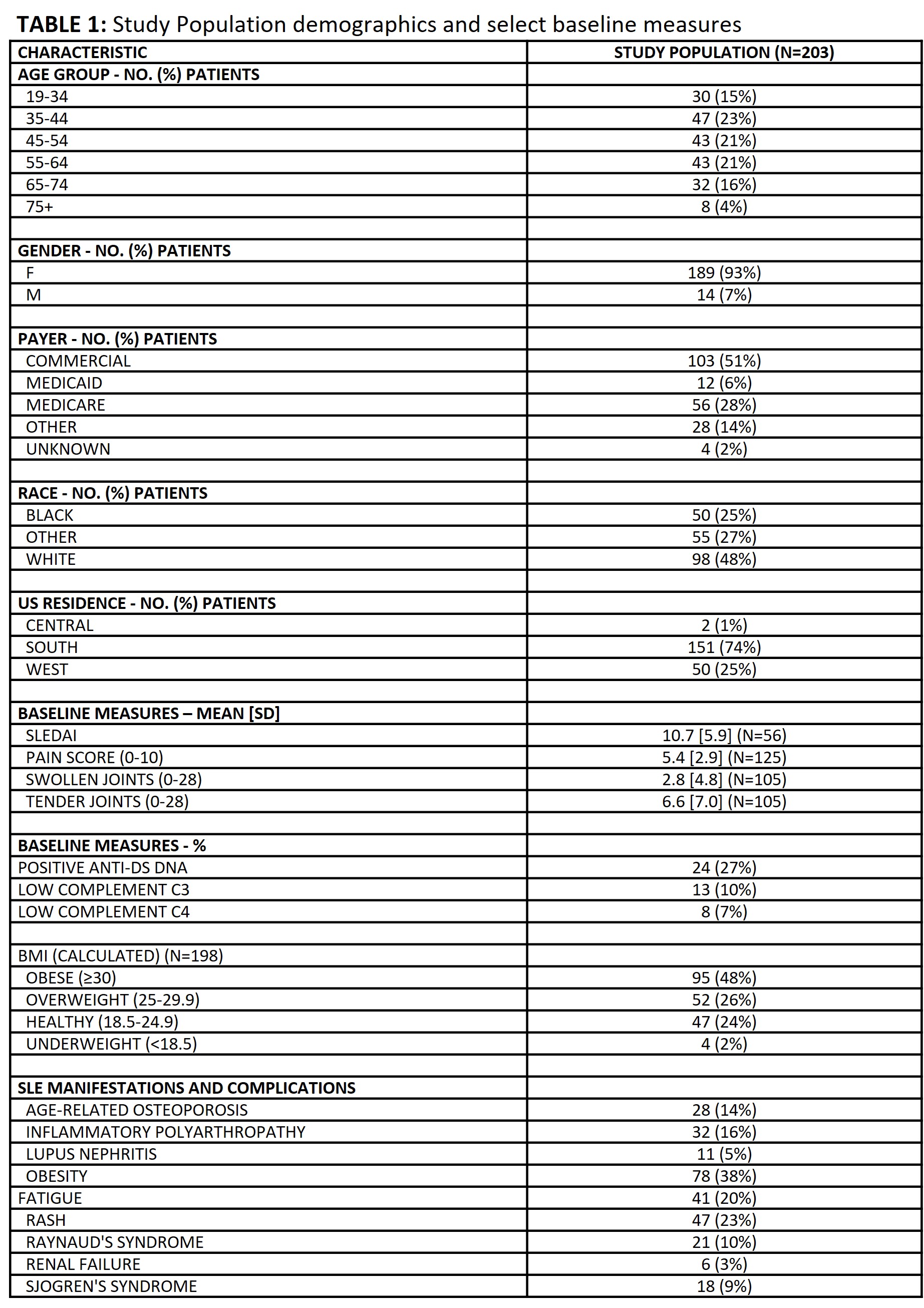
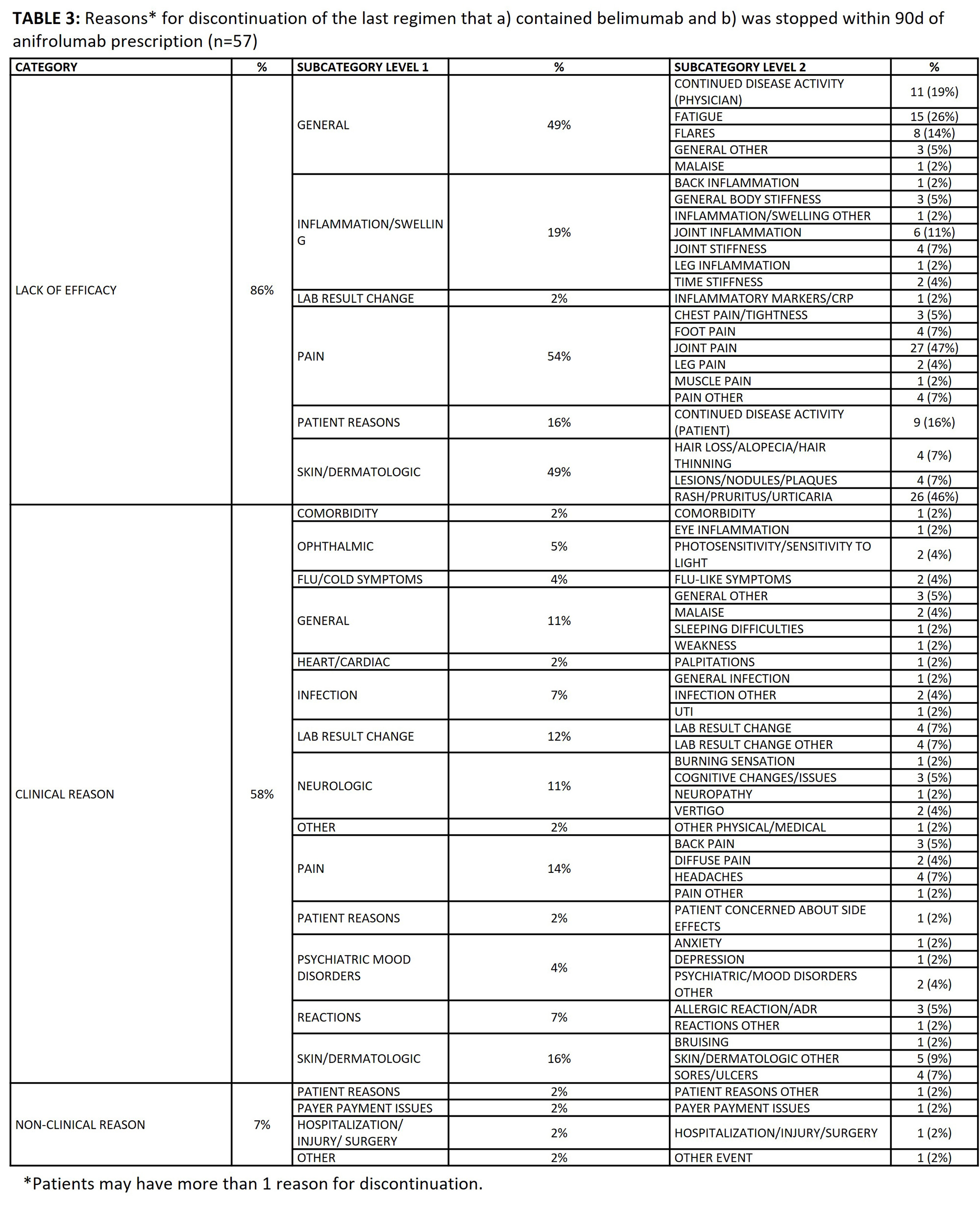
Results: 203 patients met study criteria [TABLE 1]; they were predominantly female (93%), commercially insured (51%), and in the Southern region of the U.S. (74%). At baseline, mean±SD SLEDAI was 10.7±5.9. Treatment history prior to anifrolumab prescription included oral corticosteroids (80%), hydroxychloroquine (87%), belimumab (55%), mycophenolate (37%), and methotrexate (35%). Patients’ treatment regimen immediately prior to anifrolumab included belimumab (31%), antimalarials (76%), and immunosuppressants (52%) [TABLE 2]. 30% of patients who initiated anifrolumab were biologic-naïve. In 69/203 patients, anifrolumab substituted another medication in the treatment regimen, most notably belimumab. Therefore, we analyzed reasons behind discontinuation of belimumab prior to anifrolumab. Among the 57 patients who discontinued belimumab within 90 days of anifrolumab prescription, 49 (86%) indicated lack or loss of efficacy, 33 (58%) indicated a clinical reason, and 4 (7%) indicated a non-clinical reason [TABLE 3]. The top reasons for discontinuation from belimumab among the 57 patients included joint pain (47%), rash/pruritus/urticaria (46%), and fatigue (26%).
Conclusion: This is one of the first studies to characterize real-world utilization of anifrolumab in management of SLE patients in the US. The majority of patients receiving anifrolumab within ARN were DMARD-experienced. Lack or loss of efficacy of belimumab, most frequently associated with joint pain, skin rashes, or fatigue, was a common reason for switching to anifrolumab. These findings highlight a need to understand which patients will benefit from anifrolumab as an early-line biologic therapy.
To cite this abstract in AMA style:
Kyttaris V, Atefi G, Persons D, Ambrose C, Milligan S, Wu S. Characteristics and Prior Treatment Journey of Systemic Lupus Erythematosus (SLE) Patients Who Were Prescribed Anifrolumab — Observations from the American Rheumatology Network (ARN) in the U.S [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/characteristics-and-prior-treatment-journey-of-systemic-lupus-erythematosus-sle-patients-who-were-prescribed-anifrolumab-observations-from-the-american-rheumatology-network-arn-in-the-u-s/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/characteristics-and-prior-treatment-journey-of-systemic-lupus-erythematosus-sle-patients-who-were-prescribed-anifrolumab-observations-from-the-american-rheumatology-network-arn-in-the-u-s/