Session Information
Date: Tuesday, November 10, 2015
Title: Spondylarthropathies and Psoriatic Arthritis - Clinical Aspects and Treatment Poster III: Therapy
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Over the past decade, the treatment of
PsA has improved significantly. The purpose of this study was to describe
real-world treatment patterns among PsA patients following initiation of TNF
inhibitor (TNFi) and non-biologic (MTX) therapy over time to understand if
increasing clinical evidence for the benefit of TNFi therapy has led to changes
in treatment patterns.
Methods: Using data on PsA patients from Corrona,
a national multicenter registry of RA and PsA patients, PsA patients were
identified between 1/1/2004 and 12/31/2012 and stratified into 3 cohorts; those
who had initiated TNFi monotherapy (mono), MTX monotherapy or combination (combo)
therapy (TNFi and MTX). Patients with at least 6 months of follow-up data after
first therapy initiation were included. The 3 patient cohorts were further stratified
over three different time periods (2004-2006, 2007-2009, and 2010-2012) based
on year of initiation. Baseline demographic and
clinical characteristics were assessed at drug initiation. The key outcome
measures of interest were persistence on index medication therapy; gaps in
therapy and therapy restarts; therapy changes, including treatment switches and
add-on therapy; and length of time on therapy. Persistence was defined as
continuous use of therapy over a 12-month period.
Results: There were 520 PsA patients who met
inclusion criteria. The 3 groups differed demographically in terms of baseline characteristics.
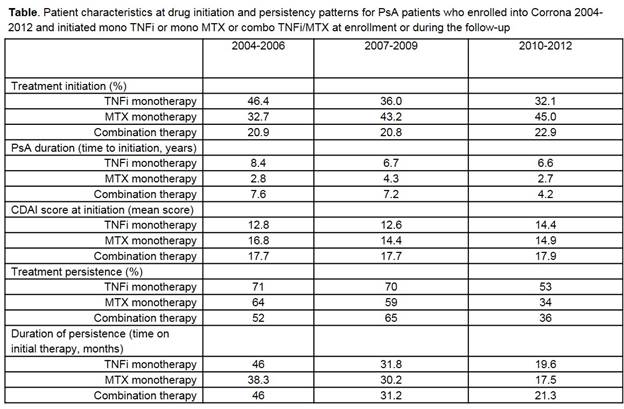
The proportion of patients initiating TNFi mono has decreased over time, while
the proportion starting combination therapy has remained steady (Table). Over
the three time periods, patients were noted to start TNFi earlier in their
disease course; however, time to initiating TNFi mono was longer than time to
initiating combo therapy. Mean CDAI at initiation was highest among the combo group
and has remained above 12 for all therapy groups in all time periods. Time on initial
therapy decreased as the time period cohorts became more contemporary, but
persistence on TNFi mono was higher than the other two cohorts across all time
periods (in 2010-2012 persistence was 53% on TNFi mono, 34% on MTX mono, and
36% on combo therapy). Patients appear less likely to remove MTX from their combo
therapy in recent years (70% in 2004-2006, 34% in 2010-2012), yet no patients
dropped TNFi from their combo therapy. In the most recent time period, 11% of
mono TNFi users became combo users, whereas 28% of mono MTX users became combo users.
Conclusion: Treatment patterns in PsA patients have
changed from 2004-2012, including earlier TNFi initiation and more cycling.
This may be due to the increasing number of treatment options available and
increased focus on achievement of low disease activity state. However, physicians
do not appear to be more likely to initiate patients on TNFi monotherapy even
though the clinical evidence supporting their effectiveness has increased over
this same time period and patients remain more persistent with it.
To cite this abstract in AMA style:
Mease PJ, Lesperance T, Accortt N, Collier D, Liu M, Mason M, Deveikis S. Changes in Treatment Patterns in Psoriatic Arthritis Patients Newly Initiated on Biologic and Non-Biologic Therapy Enrolled in a North American Clinical Registry [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/changes-in-treatment-patterns-in-psoriatic-arthritis-patients-newly-initiated-on-biologic-and-non-biologic-therapy-enrolled-in-a-north-american-clinical-registry/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/changes-in-treatment-patterns-in-psoriatic-arthritis-patients-newly-initiated-on-biologic-and-non-biologic-therapy-enrolled-in-a-north-american-clinical-registry/