Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Changes in demographics of study population enrolled in long-term osteoporosis clinical trials may affect interpretation of efficacy/safety outcomes. Denosumab is being evaluated for up to 10 years in the 3-year FREEDOM trial and 7-year Extension. Denosumab for up to 8 years is associated with low fracture incidence, continued BMD increases, and adverse event profile similar to what has previously been reported (Papapoulos et al., JBMR. 2013;28:S1). We compared the FREEDOM and Extension populations at baseline and in the Extension to assess potential selection bias that might influence long-term treatment outcomes.
Methods: In FREEDOM, women were randomized to placebo or denosumab 60 mg every 6 months. All FREEDOM subjects who had not missed >1 dose of investigational product, completed the 3-year visit, and consented to enroll were eligible to receive open-label denosumab in the Extension. We assessed whether older age and incident fractures contributed to attrition at Year 5 of the Extension, representing 8 years of follow-up.
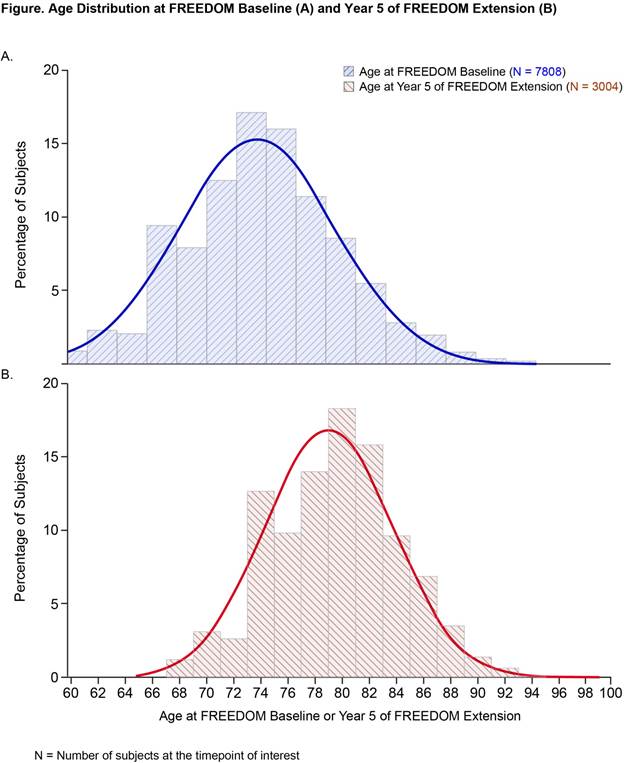
Results: In FREEDOM, 6478/7808 (83%) subjects completed the trial. Of 5928 subjects eligible for the Extension, 4550 (77%) enrolled. Through Year 5 of the Extension, 3004 (66%) remained on study. While baseline characteristics were similar in FREEDOM and Extension, all subjects were 3 years older at Extension baseline, prevalent vertebral fracture rate in placebo-treated subjects was higher at Extension baseline compared with FREEDOM baseline (25% vs 22%), and denosumab-treated subjects had higher mean BMD at Extension baseline. Age distribution after 5 years of Extension remained consistent with the antecedent 3 years in FREEDOM, with no preponderance of younger subjects (Figure). As expected, older subjects were more likely to discontinue, however, 62% of subjects who were ≥75 years at Extension baseline remained on study through Year 5. While subjects who fractured were more likely to discontinue in FREEDOM and in the Extension, 88% and 83% of placebo and denosumab fractured subjects, respectively, completed the 3-year FREEDOM trial, and 72% of fractured subjects in the Extension remained enrolled through Year 5.
Conclusion: In this large-scale, long-term study of denosumab, Extension population maintained similar characteristics to the original FREEDOM cohort. During Extension, a high percentage of subjects at increased risk for fractures due to older age and incident fracture remain on study. This suggests that the low fracture incidence and consistent safety profile reflect the long-term denosumab treatment effect.
Disclosure:
J. Adachi,
Amgen Inc., Eli Lilly, Merck,
2,
Amgen Inc., Eli Lilly, Merck, Warner Chilcott,
5,
Amgen Inc., Eli Lilly, Warner Chilcott,
8;
C. Lin,
Amgen Inc.,
1,
Amgen Inc.,
3;
P. Ho,
Amgen Inc.,
1,
Amgen Inc.,
3;
M. Bolognese,
Amgen Inc., Regeneron, Lilly,
2,
Amgen Inc.,
8;
H. Bone,
Amgen Inc., Merck, Novartis, NPS,
2,
Amgen Inc., Merck, Novartis, Tarsa, Noven,
5;
P. Hadji,
Amgen Inc., Elli Lilly,
5,
Amgen Inc., Elli Lilly,
8;
S. Papapoulos,
Amgen Inc., Merck & Co, Novartis, Axsome, Gador,
5,
International Osteoporosis Foundation,
6,
Amgen Inc., GSK, Novartis, Roche,
8;
C. Recknor,
None;
N. Daizadeh,
Amgen Inc.,
1,
Amgen Inc.,
3;
P. Dakin,
Amgen Inc.,
1,
Amgen Inc.,
3;
R. Wagman,
Amgen Inc.,
1,
Amgen Inc.,
3;
S. Ferrari,
Amgen Inc., MSD,
2,
Amgen Inc., MSD, Lilly, GSK, Bioiberica,
5.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/changes-in-subject-characteristics-in-the-denosumab-pivotal-fracture-trial-and-its-extension-for-up-to-8-years/