Session Information
Date: Sunday, November 5, 2017
Title: Spondyloarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment I
Session Type: ACR Concurrent Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: Cardiovascular (CV) disease and cardiometabolic syndrome are common comorbidities/causes of mortality in patients (pts) with psoriatic arthritis (PsA). Tofacitinib is an oral Janus kinase inhibitor under investigation for PsA. We investigated changes in lipid levels and incidence of CV events in pts with PsA treated with tofacitinib in Phase (P) 3 and long-term extension (LTE) studies.
Methods: Data were analyzed for pts who received ≥1 dose of tofacitinib 5 or 10 mg BID or placebo (PBO), integrated across 2 P3 studies (OPAL Broaden [12 months (mo); NCT01877668, including adalimumab control]; OPAL Beyond [6 mo; NCT01882439]) and 1 LTE study (OPAL Balance [data cut-off May 2016; ongoing, database not locked; NCT01976364]). Lipid levels were assessed throughout P3 and LTE studies; this analysis included data from the PBO-controlled period (Mo 0–3) of P3 studies. Blood pressure, hypertension events (standardized MedDRA query [narrow]), and adjudicated (independent/blinded to treatment) major adverse cardiovascular events (MACE) are reported for all pts who received ≥1 dose of tofacitinib, pooled across doses. Incidence rates (IR; pts with events/100 pt-years [PY]) and 95% CI are reported.
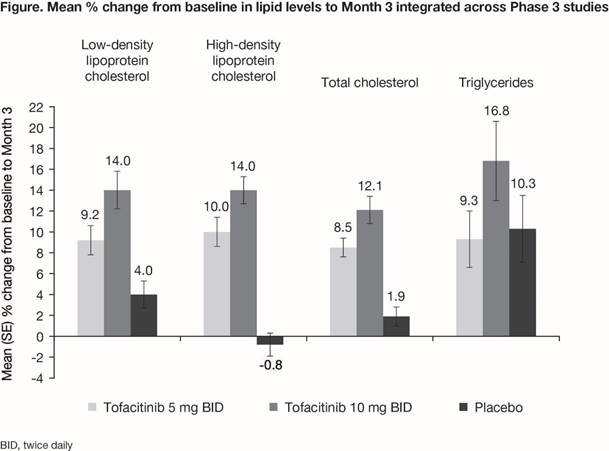
Results: Overall, 783 pts (776 PY of tofacitinib exposure) were included in P3 and LTE studies; treatment duration was 1–927 days. After 3 mo of tofacitinib treatment in P3 studies, dose-dependent increases in lipid levels were observed with tofacitinib; minimal changes were observed with PBO, except for triglycerides (Fig). Concurrent increases in high-density and low-density lipoprotein (HDL/LDL) and no change in the total cholesterol/HDL ratio were shown. Across P3 and LTE studies, no clinically significant changes in systolic or diastolic blood pressure were seen to 24 mo. Hypertension events were reported in 38 (4.9%) pts: IR 4.93 (95% CI 3.49, 6.77). Of these events, 4 led to pt discontinuation, and 2 were serious adverse events. MACE were reported for 3 (0.4%) pts receiving tofacitinib (IR 0.38; 95% CI 0.08, 1.11), and included sudden cardiac death (57 days of exposure at time of event), myocardial infarction (197 days), and ischemic stroke (80 days). This is within the range reported in tofacitinib studies in pts with psoriasis (IR 0.24 [0.15, 0.37]; 8,759 PY of exposure) and rheumatoid arthritis (RA) (IR 0.38 [0.30, 0.47]; 21,886 PY of exposure). No dose-dependent effects on blood pressure, hypertension, or MACE were apparent.
Conclusion: In pts with PsA, the magnitude and dose dependency of increases in lipid levels to Mo 3 were consistent with findings in tofacitinib studies in pts with psoriasis and RA. In P3 and LTE studies, no clinically significant changes were seen in blood pressure or incidence of hypertension. Incidence of MACE was within the range reported in prior tofacitinib studies in psoriasis and RA; however, the long latency of MACE requires longer-term observation.
To cite this abstract in AMA style:
Gladman DD, Charles-Schoeman C, McInnes IB, Veale DJ, Thiers B, Graham D, Wang C, Jones T, Wolk R, DeMasi R. Changes in Lipid Levels and Incidence of Cardiovascular Events Following Tofacitinib Treatment in Patients with Psoriatic Arthritis: An Integrated Analysis across Phase 3 and Long-Term Extension Studies [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/changes-in-lipid-levels-and-incidence-of-cardiovascular-events-following-tofacitinib-treatment-in-patients-with-psoriatic-arthritis-an-integrated-analysis-across-phase-3-and-long-term-extension-studi/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/changes-in-lipid-levels-and-incidence-of-cardiovascular-events-following-tofacitinib-treatment-in-patients-with-psoriatic-arthritis-an-integrated-analysis-across-phase-3-and-long-term-extension-studi/