Session Information
Date: Sunday, October 21, 2018
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Tofacitinib is an oral Janus kinase inhibitor for the treatment of psoriatic arthritis (PsA) and rheumatoid arthritis (RA). In most countries where tofacitinib is approved, 5 mg twice daily (BID) is the only recommended dose for PsA and RA. An important component of any product labeling is information on the need for laboratory monitoring. This post hoc analysis aimed to provide information on the effect of tofacitinib 5 mg BID on laboratory values in PsA and RA patients (pts).
Methods: For analysis of pts with active PsA treated with tofacitinib 5 mg BID, data were pooled from 2 Phase 3 studies and an ongoing long-term extension (LTE) study (data cut-off, January 25, 2017; database not locked; data may change). For analysis of pts with moderate or severe RA treated with tofacitinib 5 mg BID, data were pooled from 8 Phase 2, 7 Phase 3, and 1 LTE studies (data cut-off, March 2, 2017 for LTE; database not locked; data may change). All PsA and most RA pts received a background conventional synthetic DMARD. Data (to Month 12) for pts receiving constant tofacitinib 5 mg BID were evaluated, comprising pts who received tofacitinib 5 mg BID across studies, either at randomization or following switch from placebo. Pts in the placebo groups who switched to tofacitinib 5 mg BID at Month 3 were included from the time they first received tofacitinib. Pts who switched tofacitinib dose were excluded. Change from baseline in hematologic (hemoglobin, neutrophils, lymphocytes) and lipid (LDL-cholesterol, HDL-cholesterol, total cholesterol, triglyceride) levels and key liver tests (bilirubin, ALT, AST) were analyzed. Although not addressed in the product labeling, creatine kinase, creatinine, and C-reactive protein levels were also assessed. Pts meeting protocol-defined discontinuation criteria for laboratory values were evaluated.
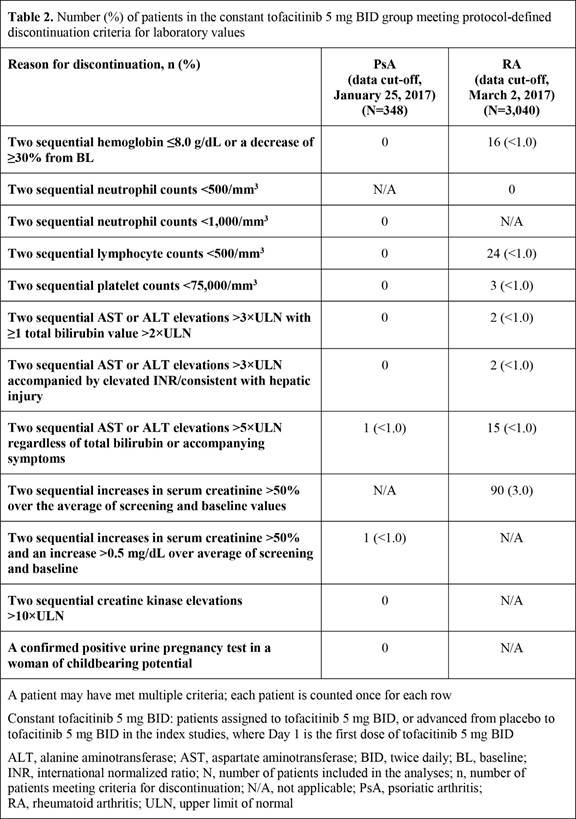
Results: The constant tofacitinib 5 mg BID group comprised 348 PsA pts and 3,040 RA pts. Table 1 presents mean/percentage changes (SE) from baseline for laboratory values. Following initial increases/reductions, key laboratory values remained stable to Month 12 in both PsA and RA, except for a reduction in lymphocyte levels, which stabilized at later time points (data not shown). In both PsA and RA, ≤3.0% of patients met discontinuation criteria for any laboratory values (Table 2).
Conclusion: In this post hoc analysis of laboratory data with tofacitinib 5 mg BID, changes in key laboratory values were similar for PsA and RA, and discontinuations due to protocol criteria being met for laboratory values were infrequent. These results provide further information on the effect of tofacitinib on laboratory values in PsA and RA.
To cite this abstract in AMA style:
Rigby WF, Burmester GR, FitzGerald O, Azevedo VF, Nash P, Hendrikx T, Graham D, Wang C, Jones T. Changes in Key Laboratory Values with Tofacitinib 5mg BID Treatment in Patients with Psoriatic Arthritis and Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/changes-in-key-laboratory-values-with-tofacitinib-5mg-bid-treatment-in-patients-with-psoriatic-arthritis-and-rheumatoid-arthritis/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/changes-in-key-laboratory-values-with-tofacitinib-5mg-bid-treatment-in-patients-with-psoriatic-arthritis-and-rheumatoid-arthritis/