Session Information
Date: Sunday, November 13, 2016
Title: Rheumatoid Arthritis – Human Etiology and Pathogenesis - Poster I
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
BACKGROUND/PURPOSE:
Protein citrullination, the post-translational conversion of arginine to citrulline, mediated by peptidylarginine deiminase (PAD) enzymes, is considered a likely mechanism for the stimulation of anti-citrullinated protein antibodies (ACPA) in patients with rheumatoid arthritis (RA). Hypercitrullination, the citrullination of multiple intracellular proteins, was recently demonstrated in synovial fluid (SF) cells from RA patients (Romero et al.Sci Transl Med, 2013). This unique form of citrullination is proposed to occur via immune-mediated, pore-forming membranolytic pathways such as perforin-granzyme activation, but is not found in other physiological processes involving citrullination, such as NETosis. Indeed, recent findings demonstrate perforin and granzyme-producing CD8+T effector cells in the synovium of pre-clinical RA patients as well as in the peripheral blood (PB) and SF of active RA patients, and persisting into disease remission.
Insight into the existence of potential cytotoxic mechanisms occurring in RA comes from the co-occurrence of RA in up to 36% of cases of T-cell Large Granular Lymphocyte (T-LGL) Leukemia, a clonal condition characterized by severe neutropenia attributed to the killing of neutrophils or their precursors by cytotoxic CD8+ T cells. Interestingly, CD8+T cells with features of LGLs were found in the SF of RA patients without LGL leukemia, but not in the SF of psoriatic arthritis and ankylosing spondylitis patients. We thus decided to test LGL leukemia as a model for neutrophil-directed cytotoxicity contributing to hypercitrullination and disease propagation in inflamed joints of ACPA+ RA patients.
METHODS:
The sera of 11 T-LGL leukemia patients (T-LGL-RA) and 15 T-LGL leukemia patients with co-existing RA (T-LGL+RA) with absolute neutrophil count (ANC) < 500 were analyzed for ACPA positivity by ELISA. ACPA titers and ANC of each group of patients were compared using unpaired two-tailed T tests. Hypercitrullination in PB neutrophils was analyzed by immunoblotting electrophoresed neutrophil cell lysates using a broad-spectrum anti-citrulline antibody. Ionomycin was used as a positive control for hypercitrullination.
RESULTS:
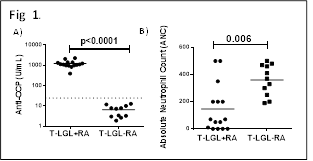
All T-LGL+RA were ACPA positive, in stark contrast to the T-LGL patients without co-existing RA (Fig. 1A). Of interest, T-LGL+RA patients had decreased ANC (Fig. 1B) as compared to T-LGL-RA patients, supporting the hypothesis of neutrophil destruction in development of ACPA positivity. Importantly, hypercitrullination was observed in neutrophils isolated from both RA and T-LGL+RA patients.
CONCLUSION:
These results are consistent with the hypothesis that in T-LGL+RA, ACPA generation may result from neutrophil-directed lysis and hypercitrullination. Elucidating the cause of neutrophil-directed cytotoxicity may help to uncover a mechanism for hypercitrullination, ACPA formation and RA disease propagation.
To cite this abstract in AMA style:
Gazitt T, Lood C, Sun X, Feith D, Ledbetter J, Starkebaum G, Loughran T Jr., Elkon KB. Cell-Mediated Neutrophil Lysis-a Mechanism Promoting Hypercitrullination in Rheumatoid Arthritis? [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/cell-mediated-neutrophil-lysis-a-mechanism-promoting-hypercitrullination-in-rheumatoid-arthritis/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cell-mediated-neutrophil-lysis-a-mechanism-promoting-hypercitrullination-in-rheumatoid-arthritis/