Session Information
Date: Tuesday, November 15, 2016
Title: Rheumatoid Arthritis – Small Molecules, Biologics and Gene Therapy - Poster III
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients with rheumatoid arthritis (RA) are at elevated risk for cardiovascular (CV) disease. Patients using tocilizumab (TCZ) may experience increased serum lipid levels. It is not known whether TCZ affects the risk for CV events relative to tumor necrosis factor inhibitors (TNFi) in patients with RA.
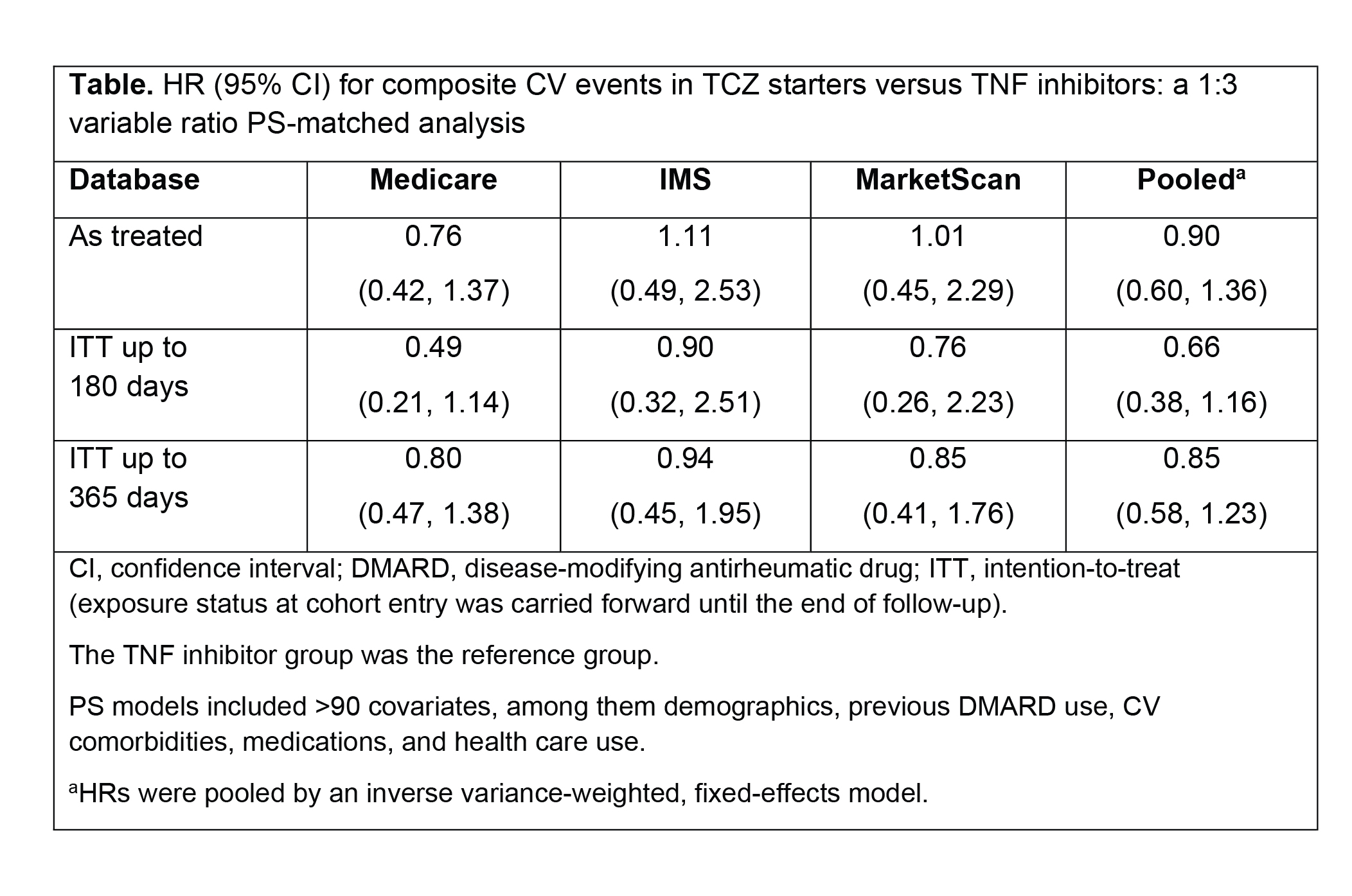
Methods: To examine comparative CV safety, we conducted a cohort study of RA patients who newly started TCZ or a TNFi using claims data from Medicare (2010-2013) or commercial health insurance (IMS Health, 2011-2014; MarketScan, 2011-June 2015). All patients were required to have previously used a different TNFi, abatacept, or tofacitinib. The primary outcome was a composite CV end point of nonfatal myocardial infarction and stroke based on a validated claims-based algorithm of high specificity (positive predictive value >94%). For the primary as-treated analysis, follow-up time started the day after TCZ or TNFi initiation and ended on treatment discontinuation plus 30 days, outcome occurrence, disenrollment, death, or end of the study. To control for >90 potential confounders including demographics, previous DMARD use, CV comorbidities, medications, and health care use, TCZ starters were propensity score (PS) matched to TNFi starters, with a variable ratio of 1:3 within each database. We estimated the incidence rate (IR) of composite CV events in the TCZ group compared with the TNFi group separately in each database. Hazard ratios (HRs) from the three PS-matched cohorts were pooled by an inverse variance-weighted, fixed-effects model.
Results: We included 8790 TCZ starters PS matched to 17,821 TNFi starters in all three databases. Mean age of the patients was 72 years in Medicare, 51 in IMS, and 53 in MarketScan. More than 80% of patients were women. At baseline, 72% (Medicare), 73% (IMS), and 66% (MarketScan) of patients used methotrexate. In the as-treated analysis, the median follow-up time varied between 176 days (Medicare) to 205 days (MarketScan) in the TCZ group and 206 days (Medicare) to 231 days (MarketScan) in the TNFi group. Across the three databases, 35 CV events occurred in the TCZ group and 80 occurred in the TNFi group. The IR of composite CV events per 100 person-years ranged from 0.31 (MarketScan) to 0.87 (Medicare) in the TCZ group and from 0.31 (MarketScan) to 1.09 (Medicare) in the TNFi group. The risk for CV events was similar between TCZ and TNFi users across all three databases (Table), with a pooled HR of 0.90 (95% CI, 0.60-1.36) from the as-treated analysis. Adjustment for a potential overlap between the commercial cohorts (IMS and MarketScan) yielded consistent results.
Conclusion: This large multi-database cohort study found no increase in the rate of CV events in patients with RA who switched from a biologic agent to either TCZ or a different TNFi.
To cite this abstract in AMA style:
Kim SC, Solomon DH, Rogers JR, Gale S, Klearman M, Sarsour K, Schneeweiss S. Cardiovascular Safety of Tocilizumab Versus Tumor Necrosis Factor Inhibitors in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/cardiovascular-safety-of-tocilizumab-versus-tumor-necrosis-factor-inhibitors-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cardiovascular-safety-of-tocilizumab-versus-tumor-necrosis-factor-inhibitors-in-patients-with-rheumatoid-arthritis/