Session Information
Date: Sunday, October 21, 2018
Title: Rheumatoid Arthritis – Treatments Poster I: Strategy and Epidemiology
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The red blood cell (RBC) Methotrexate (MTX) polyglutamate (MTXPG3) assay is a helpful therapeutic drug monitoring (TDM) tool in autoimmune rheumatic diseases. Our objective was to transition the RBC MTXPG assay from venous blood to capillary blood collected by fingerstick.
Methods: Adult subjects with Rheumatoid Arthritis (RA) (mean age 62±13 [SD]) treated with low dose weekly MTX therapy (mean dose 17±5 mg/week [SD]) were enrolled from two rheumatology practices in the United States. Clinical staff consented subjects and collected paired specimens: a capillary blood specimen (10µl) collected by fingerstick on volumetric absorptive microsampling device (Neoteryx, Torrance, CA, USA), and a venous blood specimen (10 ml) collected in EDTA containing tubes. Dried capillary blood and venous blood were shipped overnight to a CAP/CLIA accredited clinical laboratory. RBC MTXPG3 levels from capillary and venous blood were measured using validated liquid chromatography coupled with high sensitivity tandem mass spectrometry. Patient reported outcomes (PROs) comparing venipuncture and fingerstick collection methods were obtained at the time of the visit. RBC MTXPG3 levels from capillary blood were compared to historical levels estimated from a database of venous blood specimens collected from patients on MTX therapy and assessed for compliance, defective activation to polyglutamates (RBC MTXPG3 <5 nmol/L), or inadequate response to MTX.
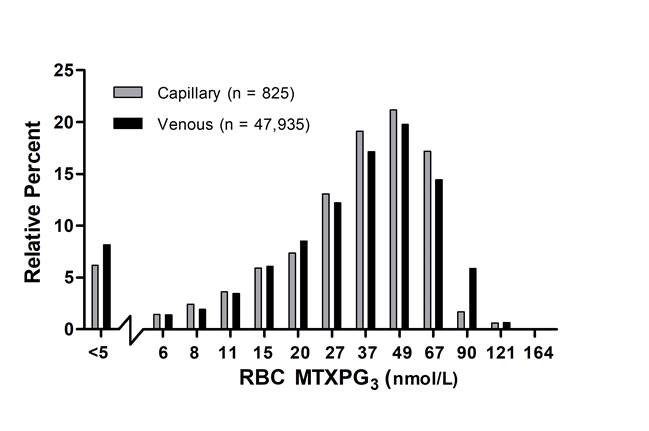
Results: In the 106 RA subjects enrolled, mean (SD) RBC MTXPG3 levels recovered from dried capillary blood and venous blood specimens were similar (30±18 nmol/L vs 33±19 nmol/L, respectively) (R2=0.89; slope=1.10). PROs indicated that the fingerstick collection method was convenient and non-inferior to venipuncture, but only a minority of subjects (37%) were comfortable with self-collection of the capillary blood specimen at home. Following one year of testing, capillary RBC MTXPG3 (36±20 nmol/L RBC) levels from a population of 679 patients (mean age 58±15 years; n=825 capillary blood specimens) were similar to RBC MTXPG3 (38±24 nmol/L) levels estimated from the database of patient specimens collected using venipuncture (mean patient age 58±15 years; n=47,935). Potential for poor compliance (RBC MTXPG3≤5 nmol/L RBC) was detected in 7.0% (n=54) and 8.5% (n=4,224) patient specimens collected using the fingerstick and venipuncture based method, respectively (Figure).
Conclusion: Measurement of capillary blood MTXPG3 levels can be applied to the TDM of MTX in clinical rheumatology practice. PROs suggest that patient training will be required before transitioning from office-based to home-based specimen collection.
To cite this abstract in AMA style:
Dervieux T, Brady K, Stimson D, Qu Y, O'Malley T, Apilado R, Reddy S, Chitkara P, Conklin J, Alexander R, Ibarra C. Capillary Blood Based Methotrexate Polyglutamate Assay for Monitoring Low Dose Methotrexate Therapy in Rheumatic Diseases [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/capillary-blood-based-methotrexate-polyglutamate-assay-for-monitoring-low-dose-methotrexate-therapy-in-rheumatic-diseases/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/capillary-blood-based-methotrexate-polyglutamate-assay-for-monitoring-low-dose-methotrexate-therapy-in-rheumatic-diseases/