Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Recurrent fever and serositis are the hallmarks of Familial Mediterranean fever (FMF). Colchicine is known for yielding high success in disease control by obviating attacks; however, management of the disease may be challenging in the case of colchicine resistance. In this specific group, which consists 5-10% of FMF patients, induction of biological agents targeting inflammatory pathways may be crucial and often provides rewarding results. The aim of the survey was to evaluate the long-term efficacy of the canakinumab treatment in colchicine-resistant FMF patients followed-up in a single center.
Methods: Medical records of patients, who were diagnosed as FMF and routinely being followed-up in a tertiary rheumatology center experienced in auto-inflammatory diseases, were analyzed retrospectively. Colchicine-resistant FMF patients without amyloidosis who received at least one dose of canakinumab were included in the survey. All of the patients fulfilled the diagnostic criteria for FMF. Colchicine resistance was defined as one or more typical attacks in a month despite having maximum tolerated dose of colchicine compliantly. Attack frequency, patient global assessment scores (0-10 Visual Analogue Scale [VAS]), and measurement of acute phase reactant levels were utilized for evaluating disease severity before and after the induction of canakinumab therapy. Baseline characteristics, events leading to treatment discontinuation, and the time elapsed from discontinuation to the first flare were registered.
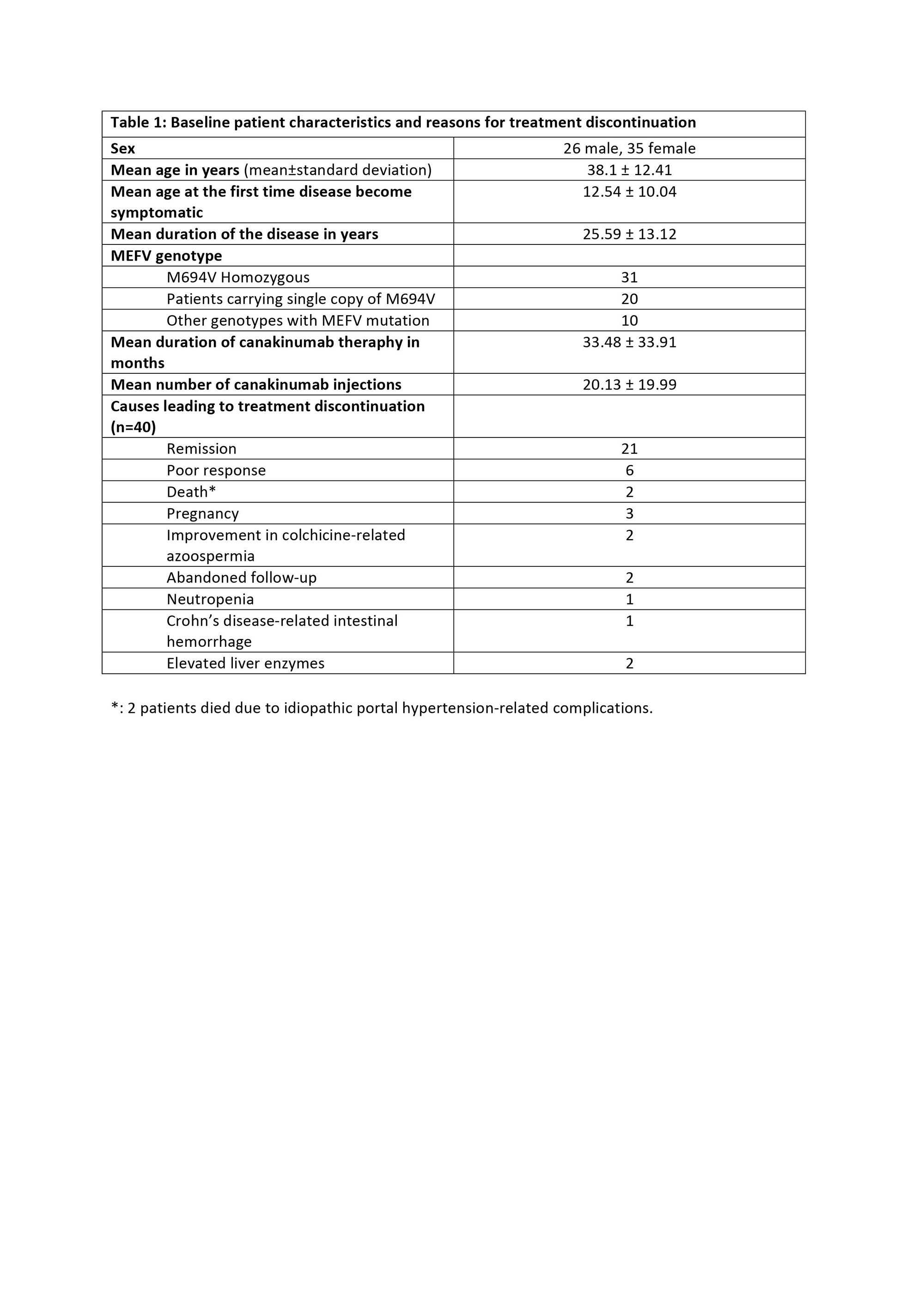
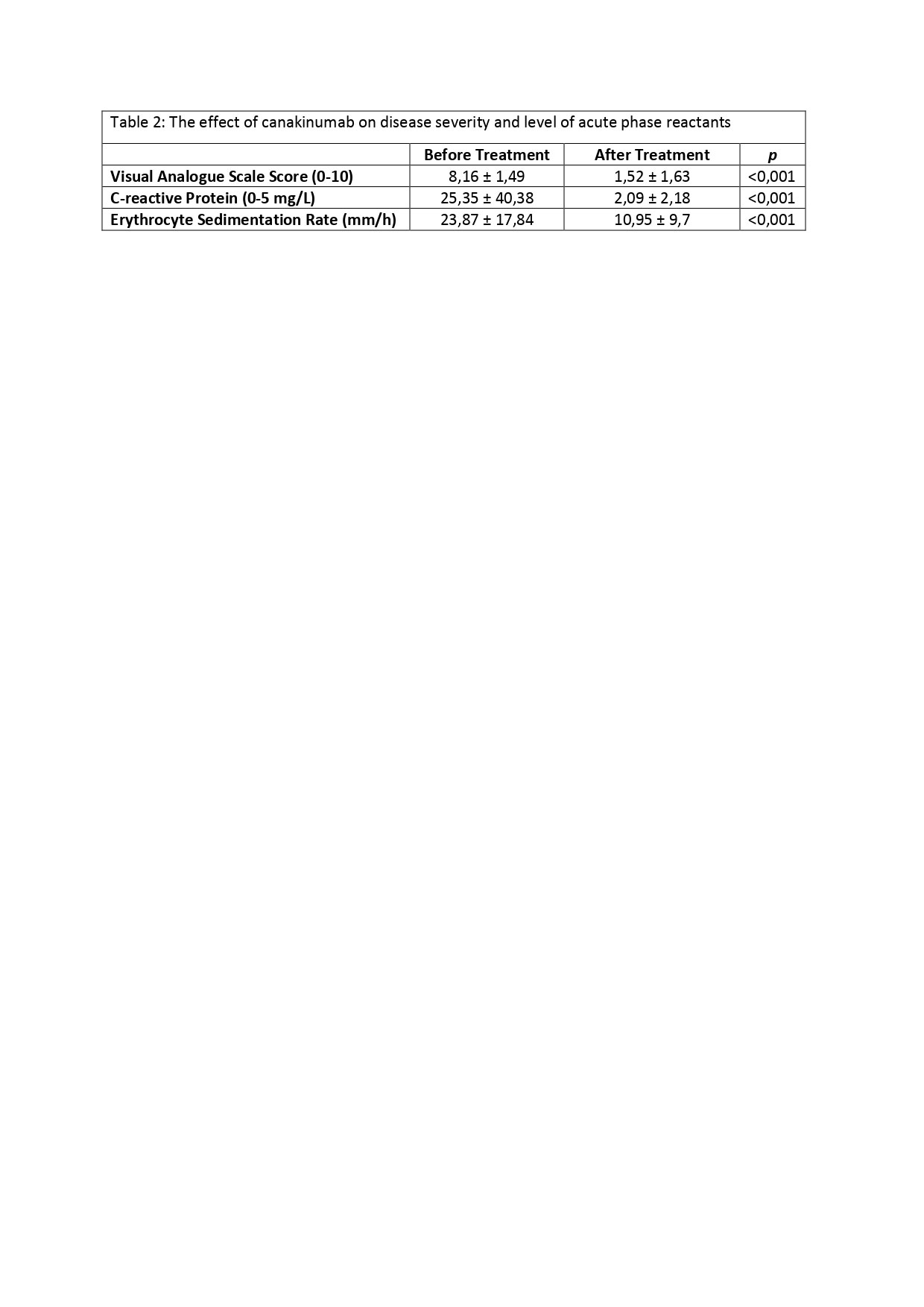
Results: Baseline characteristics of 61 patients consisting the study population and reasons for treatment discontinuation are displayed in Table 1. 21 out of 61 patients (34.42%) described no attacks after canakinumab therapy, hence those were classified as the patients in remission. Attack frequency decreased at least 50% in 34 patients (55.73%). 6 patients (9.83%) had an unsatisfactory response to the treatment; they failed to accomplish at least 50% decrease in the attack frequency and ended up with treatment termination. Canakinumab therapy was also discontinued in patients who had undergone remission; 16 out of 21 (76.19%) experienced a flare within 3.27 ± 1.95 months following the treatment discontinuation, and canakinumab had to be prescribed again in 13 of the 16 patients. 4 patients (19.05%) reported no flare, and 3 of the 4 patients are currently treated with only colchicine. Status of the remaining 1 patient regarding the flare-up is unknown due to patient’s non-compliance to follow-ups. In all cohort, 40 patients are still receiving canakinumab therapy. VAS scores and acute phase reactant levels decreased remarkably after the treatment (p< 0.001) (Table 2)
Conclusion: Canakinumab provides a significant reduction in disease severity, attack frequency, and acute phase reactant levels. Based on our real-life experience, canakinumab therapy proves its value in the treatment of colchicine-resistant FMF patients by improving patient-reported outcomes and the degree of subclinical inflammation. Canakinumab is a safe and efficient treatment modality for long-term follow-up.
To cite this abstract in AMA style:
Durucan I, Ayla A, Besiroglu H, Alkan A, Selvi O, Ozdogan H, Ugurlu S. Canakinumab Treatment in Familial Mediterranean Fever Patients with Colchicine Resistance: A Single-center Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/canakinumab-treatment-in-familial-mediterranean-fever-patients-with-colchicine-resistance-a-single-center-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/canakinumab-treatment-in-familial-mediterranean-fever-patients-with-colchicine-resistance-a-single-center-study/