Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: For ethical reasons, modern phase-III RCTs in rheumatoid arthritis (RA) have limited placebo exposure time, resulting in uncertainties when interpreting low frequency adverse events (AEs). We developed and implemented a novel method for providing contextual AE rates through coordinated analyses of 5 RA registries.
Methods: Participating observational registries included CORRONA (USA), CORRONA International (East Europe, India, Latin America), IORRA (Japan), NOAR (UK), and SRR (Sweden). By harmonizing AE definitions in a typical RA clinical trial program and corresponding available outcomes from the registries we defined specific outcomes in 4 areas: mortality, cardiovascular disease (CVD), infection, and malignancy. Each registry defined a primary cohort (all patients included from January 2000) for optimal sample size. To address comparability and potential bias several sub-cohorts were also defined for sensitivity analyses, based on disease activity, treatment, calendar time or duration of follow-up, and relevant RCT exclusions. Rates were standardized for the potential confounders age and sex, and in a sensitivity analysis also HAQ score to the distribution in a typical RA program.
Results: The primary cohorts included 57,251 RA patients (232,985 person-years [PY]) – mean (SD) baseline age 58.2 (13.8) years, 24.5% men, and RA duration 8.2 (11.7) years (Table 1). Some important demographic differences appeared; for example, smoking was most common in NOAR, and SRR had the highest proportion with early RA. Mean baseline HAQ and DAS28 were 0.88 (0.67) and 4.1 (1.5).
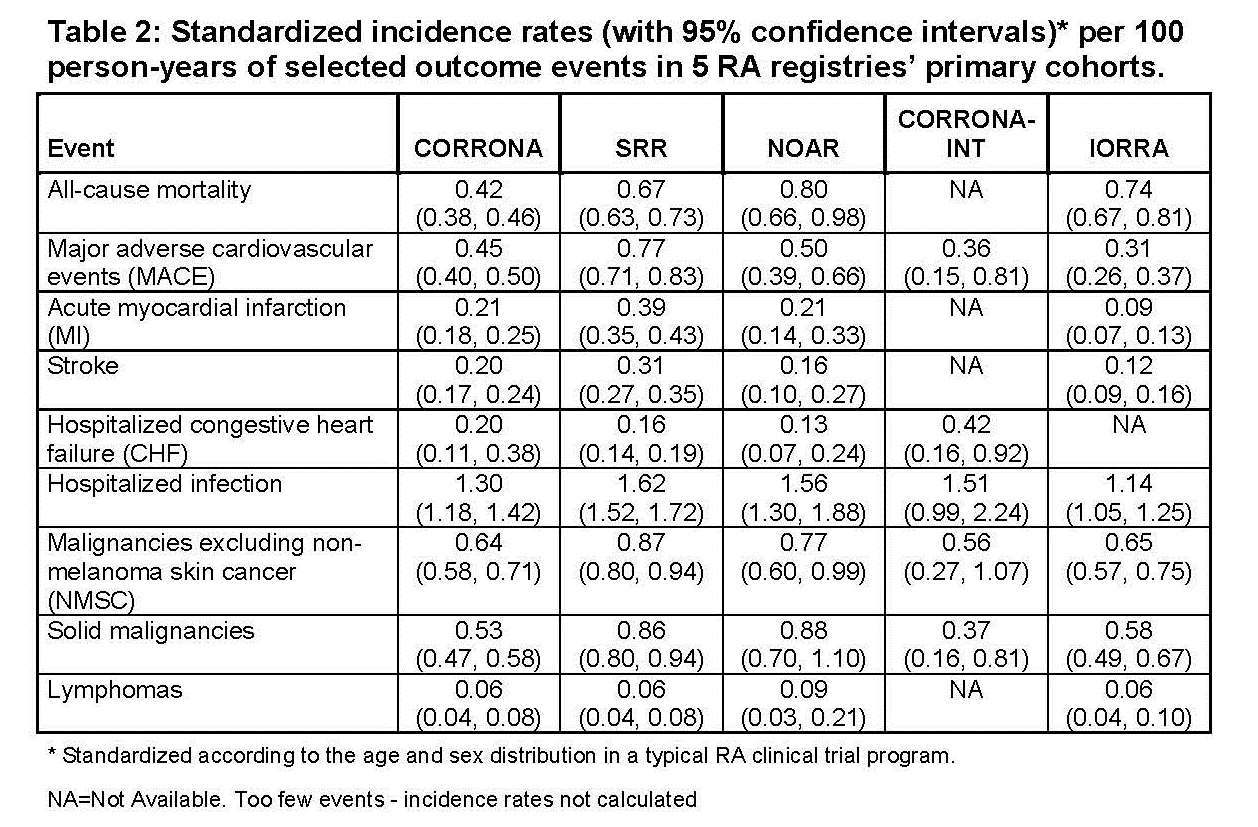
Specific outcome event rates were determined in each registry (Table 2). CVD rates varied the most with the lowest MI rate (0.09 per 100 PY) seen in IORRA and the highest (0.39) in SRR. Rates of hospitalized infections were quite consistent across registries. Sensitivity analysis returned higher mortality and MACE rates along with wider confidence intervals when standardized also for RCT HAQ levels. Additional sub-cohort analyses showed small and mostly consistent changes among registries.
Conclusion: While potential concern for bias may remain, we have successfully determined background safety rates that provide context for RCTs, through a coordinated approach between observational RA registries. This new approach may have utility to satisfy future regulatory requirements and support safety assessments.
Disclosure:
K. Michaud,
University of Nebraska Medical Center,
3,
National Data Bank for Rheumatic Diseases,
3;
J. Askling,
AstraZeneca,
2,
AstraZeneca,
5;
H. Yamanaka,
AstraZeneca, Abbott, AbbVie, Chugai, Takeda, Pfizer, Daiichi Sankyo, Mitsubishi Tanabe, Teijin, Nippon Kayaku, Taishotoyama, Bristol-Myers Squibb, Astellas, Eisai, MSD, Santen, GlaxoSmithKline, Asahikasei, Janssen,
2,
AstraZeneca, Abbott, AbbVie, Chugai, Takeda, Pfizer, Daiichi Sankyo, Mitsubishi Tanabe, Teijin, Nippon Kayaku, Bristol-Myers Squibb, Astellas, Eisai,
5,
Abbott, AbbVie, Chugai, Takeda, Pfizer, Mitsubishi Tanabe, Teijin, Bristol-Myers Squibb, Astellas, Eisai,
8;
D. Symmons,
AstraZeneca,
2,
AstraZeneca,
5;
M. Holmqvist,
None;
T. Frisell,
None;
G. Reed,
CORRONA , Inc.,
3;
D. A. Pappas,
CORRONA Inc,
3,
Novartis Pharmaceutical Corporation,
5,
Columbia University,
6;
E. Tanaka,
None;
E. Inoue,
None;
S. M. M. Verstappen,
None;
C. Garwood,
None;
L. Horne,
AstraZeneca,
3,
AstraZeneca,
1;
K. Lampl,
AstraZeneca,
3,
AstraZeneca,
1;
N. Berglind,
AstraZeneca, Bristol-Myers Squibb,
1,
AstraZeneca,
3;
S. Franzen,
AstraZeneca,
1,
AstraZeneca,
3;
F. Nyberg,
AstraZeneca,
1,
AstraZeneca,
3;
T. Tran,
MedImmune LLC,
3;
M. Ho,
AstraZeneca,
3;
J. D. Greenberg,
AstraZeneca,
5,
Corrona,
5,
Pfizer Inc,
5,
Corrona,
1.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/can-ra-registries-provide-contextual-safety-data-for-modern-rcts/