Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: Disease-specific patient reported outcome (PRO) tools are uniquely informative for the management of systemic lupus erythematosus (SLE) patients. However, it is difficult to incorporate distribution of appropriate disease specific PRO measures in busy clinical settings. A single, simple PRO measure for all patients, irrespective of their underlying disease diagnosis, can easily be integrated into the clinic check-in processes without requiring additional processes/ personnel. Multi-Dimensional Health Assessment Questionnaire (MDHAQ) is a PRO tool developed for patients with musculoskeletal diseases; RAPID 3 (Routine Assessment of Patient Index Data) scores are easily and rapidly calculated from the MDHAQ. We compared RAPID3 and LupusPRO (a disease specific PRO tool for SLE) and disease activity indices in patients with SLE seen during usual care.
Methods: 78 consenting patients meeting 4 ACR criteria for SLE completed both MDHAQ and LupusPRO during a routine clinic visit. Disease activity was assessed using SELENA-SLEDAI (Physician Global Assessment-PGA 0-3), and demographic data were obtained. We compared LupusPRO domain scores for 8 Health Related Quality of Life-HRQOL and 4 Non-Health Related Quality of Life-Non-HRQOL scales (all scored 0-100,higher scores=Better Quality of Life) and physician global assessment (DOCGL), SELENA-SLEDAI stratified by disease severity to RAPID3 scores in 4 categories,≤ 3 Remission; 3.1-6.0 Low severity; 6.1-12 Medium Severity and >12 High severity. Statistical significance was analyzed using Kruskall-Wallis non-parametric tests; a p value of ≤ 0.05 was considered significant on two tailed tests.
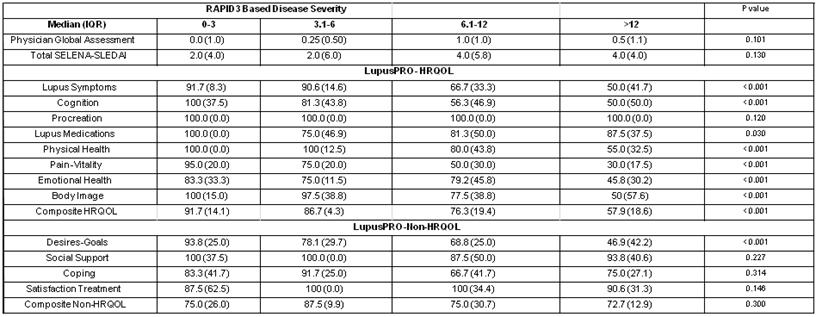
Results: Mean (SD) age, Physician global assessment (or DOCGL) and total SELENA-SLEDAI scores were 42.4 (13.8) yrs, 0.7 (0.7) and 3.9 (4.7) respectively. Mean (SD) RAPID3 score was 9.8 (7.6). Table 1 shows the median (IQR) disease activity and LupusPRO domain scores for the RAPID3 disease severity categories. Disease activity scores did not vary between RAPID3 disease severity categories. This phenomenon has been previously reported with all PRO tools (Generic or Disease Specific) used in SLE. Seven of the 12 LupusPRO domains differed significantly in patients in the 4 RAPID3 disease severity categories – lupus symptoms, cognition, physical health, pain-vitality, emotional health, body image, and desires- goals, while 5/12 domains – Procreation, Lupus Medications, Social Support, Coping and Satisfaction with Treatment, did not differ in the 4 groups. The composite HRQOL LupusPRO scores differed significantly in the RAPID3 disease severity categories, while the composite non-HRQOL did not differ.
Conclusion: RAPID3 may be used as a PRO for HRQOL in busy clinical settings to add to management of SLE patients.
TABLE 1: Disease Activity and LupusPRO scores stratified by RAPID3 Disease Severity.
Disclosure:
N. Annapureddy,
None;
T. Pincus,
None;
J. A. Block,
Ferring, Inc.,
5,
PL Pharma, Inc.,
9;
M. Jolly,
GSK,
5,
Medimmune,
5.
« Back to 2013 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/can-mdhaqrapid3-be-used-in-usual-care-of-patients-with-systemic-lupus-erythematosus/