Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: As psoriasis (Pso) commonly precedes psoriatic arthritis (PsA), an important unanswered question is whether treatment of Pso might influence the development of PsA in patients with psoriasis.
The objective of this study was to analyze the incidence of PsA in a large cohort of patients with PsO according to different treatments, with the hypothesis that treatment with biologics might prevent the development of PsA.
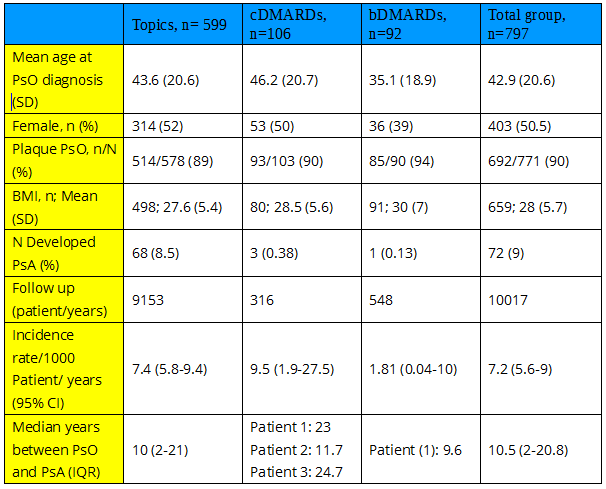
Methods: Patients with PsO without PsA followed at a University Hospital were included in this retrospective cohort study. Data was obtained from the Hospital Electronic Medical Record (EMR). Patients were classified according to their treatment in topics group (topic and phototherapy), conventional DMARDs (cDMARDs) group (Methotrexate (MTX) and cyclosporine (Cyc)), and biologic DMARDs group (bDMARDs) (TNFi, IL17i, and IL12-23i). Patients contributed time since beginning of the corresponding treatment until diagnosis of PsA, lost of follow up, end of treatment or end of study. Incident cases of PsA were attributed to one treatment if developed during the administration of that treatment and up to 6 months after its discontinuation if no other treatment was started. Incident cases that developed more than one year after discontinuation of treatment were disregarded (3 cases). Incidence rate was calculated for the whole population and for each one of the treatment groups and compared with chi2test, and rate ratios were calculated as well. A multivariable logistic model for the development of PsA was analyzed by treatment groups, adjusting by other variables.
Results: 797 patients, contributed a total of 10017 patient/years. Patient’s characteristics are shown in table 1. 599 (75%) patients were treated only with topics or phototherapy, 106 (13%) with cDMARDs ( 81% MTX and 19% Cyc) and 92 (11.5%) with biologics (TNFi: 64: etanercept: 44, adalimumab:23, infliximab:6 ; IL17i: 43: 14 Ixekizumab, 29 Secukinumab; IL12-23i: (Ustekinumab) 16; some patients received more than one biologic). During follow-up 72 patients developed PsA (68 under topics; 3 under cDMARDs (2 MTX and 1 Cyc) and 1 under biologics (1 Secukinumab): Global incidence rate: 7.2 per 1000 patient/years (table 1). Although numerically the incidence of PsA in PsO patients treated with biologics was lower, the difference was not statistically significant. In Cox regression analysis, after adjusting by sex, age, and BMI, treatment with biologics was significantly associated with a reduced risk of developing PsA: Hazard ratio (95% CI): 0.1 (0.013 – 0.7); p= 0.021.
Conclusion: Treatment with biologics in patients with PsO seemed to reduce the risk of PsA and preventing its development in this retrospective single center cohort.
*This study was achieved with a grant from PANLAR (Pan-American League Against Rheumatism).
To cite this abstract in AMA style:
Lo Giudice L, Acosta Felquer M, Mazzuoccolo L, Galimberti M, Soriano E. Can Biologics “Prevent” the Development of Psoriatic Arthritis in Psoriasis Patients? Data from a Large University Hospital Cohort in Argentina [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/can-biologics-prevent-the-development-of-psoriatic-arthritis-in-psoriasis-patients-data-from-a-large-university-hospital-cohort-in-argentina/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/can-biologics-prevent-the-development-of-psoriatic-arthritis-in-psoriasis-patients-data-from-a-large-university-hospital-cohort-in-argentina/