Session Information
Date: Tuesday, October 28, 2025
Title: (2437–2469) Systemic Lupus Erythematosus – Treatment Poster III
Session Type: Poster Session C
Session Time: 10:30AM-12:30PM
Background/Purpose: Systemic lupus erythematosus (SLE) is a chronic, multi-system autoimmune disease with heterogenous presentation. Most patients with SLE report fatigue or brain fog, which often does not respond well to standard SLE therapies. As such, even patients with well-controlled SLE often continue to have debilitating fatigue. Bupropion is a combined dopamine/norepinephrine reuptake inhibitor used in the treatment of depression. Unlike most alternative anti-depressants, bupropion has a stimulating effect, and has been shown to be effective for cancer-associated fatigue. Providers at the University of North Carolina (UNC) at Chapel-Hill Rheumatology and Nephrology clinics have been prescribing bupropion off-label for the treatment of lupus-associated fatigue for several years. The purpose of this study is to better understand patient acceptability, tolerability, and response to bupropion as a treatment for lupus-associated fatigue.
Methods: We identified patients with a lupus ICD-10 code (M32.X or L93.X), who received care at the UNC Rheumatology or Nephrology clinics and were prescribed bupropion 11/2019-10/2024. Data was manually reviewed and extracted from the electronic medical record (EMR) to confirm SLE diagnoses and to identify patients who started bupropion in this timeframe for fatigue, brain fog, poor concentration, low energy, or lack of motivation/apathy. Baseline demographic characteristics, SLE activity, mental health comorbidities, medications, and response to bupropion treatment were collected. Descriptive statistics, including means, standard deviations, and frequency distributions, were calculated.
Results: Data extraction has been completed for 34 EMRs. Mean follow-up was 2.5 yrs. The median age was 45, approximately 56% of patients identified as Black or African American, and 97% female. Over half (52%) of patients had SLEDAI-2K < 4 suggestive of low lupus disease activity. Depression (53%) and anxiety (29%) were frequent comorbidities. Thirty-two percent reported improvement in energy, fatigue, concentration, low motivation/apathy, or mood during the follow-up period. Forty-one percent currently have an active prescription for bupropion and remain on this medication. The most frequent reasons for bupropion cessation include side effects (24%), lack of efficacy (18%), and loss of initial efficacy (6%). The most frequently reported side effects included insomnia (26%), headache (12%), rash (6%), anxiety (6%), and tremor (6%).
Conclusion: Bupropion may be an efficacious treatment for SLE-associated fatigue and other cognitive symptoms with few side effects. Planned next steps include assessment of outcomes of interest in all eligible patients and utilization of efficient machine learning tools for validation, in order to reduce any errors and bias introduced by human data extraction.
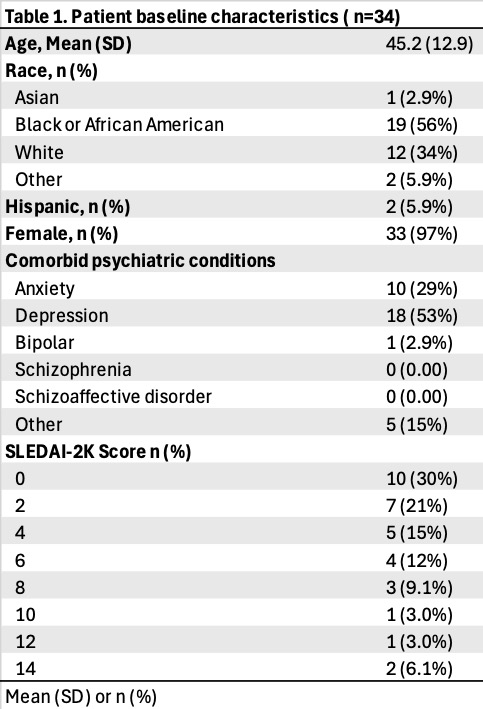 Table 1: Baseline Characteristics
Table 1: Baseline Characteristics
To cite this abstract in AMA style:
Smith A, Phan T, Luizza L, Trujillo A, Cleveland B, Saxena Beem S, Englund T, Timon C, Sheikh S. Bupropion for Lupus-Induced Fatigue Treatment (B-LIFT): A Retrospective Analysis [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/bupropion-for-lupus-induced-fatigue-treatment-b-lift-a-retrospective-analysis/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bupropion-for-lupus-induced-fatigue-treatment-b-lift-a-retrospective-analysis/

.jpg)