Session Information
Session Type: Late-Breaking Abstracts
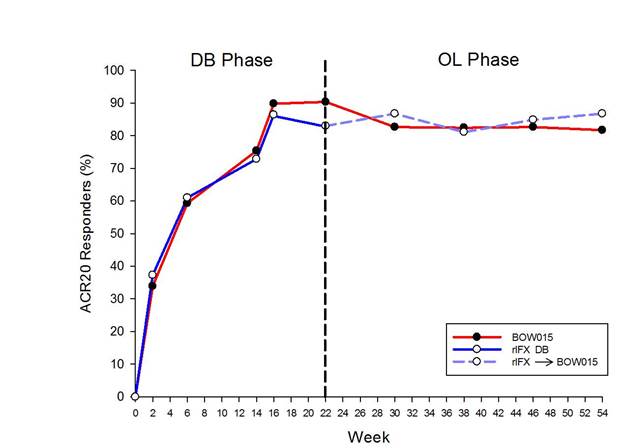
Background/Purpose: Over the first 16 wks of this phase 3 double-blind (DB) comparative effectiveness clinical trial, the comparable proportion of ACR20 responders for BOW015 (a biosimilar infliximab [IFX]) and reference IFX (rIFX) at the wk 16 primary endpoint (PE) and at each of the earlier time points provides convincing evidence of therapeutic equivalence during the steep phase of the time response curve. This 54-wk comparative effectiveness trial evaluated therapeutic equivalence of BOW015 and rIFX through wk 22 and of BOW015 continuation and switching from rIFX to BOW015 during the plateau phase by assessing efficacy, safety and immunogenicity.
Methods: 189 subjects with active RA, according to the 2010 ACR/EULAR criteria, on stable doses of oral methotrexate 10-20mg/wk were randomized 2:1 to receive either BOW015 or rIFX 3 mg/kg iv on wks 0, 2, 6, 14. 22, 30, 38 and 46. Subjects had CRP ≥10 mg/L at screening and were tested for TB by PPD, QuantiFERON-TB Gold, chest radiographs and HR-CT in selected patients. The PE was ACR20 response at wk 16. At wk 22 responders to BOW015 (BOW015-R) were continued on treatment and responders to rIFX (rIFX-R) were switched to BOW015 during an open-label (OL) phase, in which subjects were treated every 8 wks through wk 46. Efficacy and safety were assessed at each visit and immunogenicity at wks 0, 14, 30 and 58. Antibody responses to IFX were measured in two assays using a sensitive ELISA for either BOW015 or rIFX. In the OL phase, 104 BOW015-R subjects continued treatment and 53 rIFX-R subjects were switched to BOW015.
Results: ACR20 responses at 16 wks (PE) for BOW015 and rIFX, respectively, were 89.8% and 86.4% in the PP population (95% CI, [-19.3%, 12.6%]). Comparing ACR20 responses at wk 14 and wk 54 assessed the durability of the response to BOW015 over 54 wks. The ACR20 response of 72.03% for BOW015 at wk 54 was similar to that of 75.42% at wk 14.
During the double-blind (DB) phase, 52 (40.94%) BOW015-treated subjects reported 97 treatment-emergent adverse events (TEAEs) and 30 (48.39%) rIFX-treated subjects reported 45 TEAEs. 13 SAEs were reported during the DB phase: 9 (7.1%) in the BOW015-treated subjects and 4 (6.5%) in the rIFX-treated subjects. During the DB phase, 50 (48.08%) BOW015-R subjects reported 98 TEAEs and 28 (52.83%) rIFX-R subjects reported 52 TEAEs. During the OL phase, 13 SAEs were reported: 10 (6.7%) in BOW015-R subjects and 3 (5.7%) in rIFX-R subjects. No deaths were reported. At week 58, 68 (53.5%) and 35 (56.5%) of subjects initially treated with BOW015 and rIFX, respectively, had developed anti-drug antibodies.
Conclusion: This study is the first to report efficacy, safety and immunogenicity results for a biosimilar over multiple time points comparing the early kinetics and the long-term durability of the response after switching from rIFX to biosimilar BOW015. BOW015 was well tolerated and demonstrated similar immunogenicity to rIFX in both the comparative DB phase and the OL switching phase.
Disclosure:
J. Kay,
AbbVie Inc.,
2,
Eli Lilly and Company,
2,
Pfizer Inc.,
2,
Roche Laboratories, Inc.,
2,
Amgen, Inc.,
5,
AbbVie Inc.,
5,
AstraZeneca,
5,
Boehringer Ingelheim GmbH,
5,
Bristol-Myers Squibb Company,
5,
Crescendo Bioscience, Inc.,
5,
Eli Lilly and Company,
5,
Epirus Biopharmaceuticals, Inc.,
5,
Genentech Inc.,
5,
Hospira, Inc.,
5,
Janssen Biotech, Inc.,
5,
Pfizer Inc.,
5,
Samsung Bioepis,
5,
Roche Laboratories, Inc.,
5,
UCB, Inc.,
5;
M. Wyand,
Epirus Biopharmaceuticals, Inc.,
3;
S. Chandrashekara,
Epirus Biopharmaceuticals, Inc.,
9;
D. Jacob Olakkengil,
Epirus Biopharmaceuticals, Inc.,
9;
K. Bhojani,
Epirus Biopharmaceuticals, Inc.,
9;
G. Bhatia,
Epirus Biopharmaceuticals, Inc.,
9;
G. Rathi,
Epirus Biopharmaceuticals, Inc.,
9;
S. Maroli,
Epirus Biopharmaceuticals, Inc.,
5;
E. Thomson,
Epirus Biopharmaceuticals,
5;
C. Lassen,
Epirus Biopharmaceuticals,
3;
L. Shneyer,
Epirus Biopharmaceutical,
5,
Merck Pharmaceuticals,
1;
A. Chopra,
Epirus Biopharmaceuticals, Inc.,
2,
Epirus Biopharmaceuticals, Inc.,
5.
« Back to 2014 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bow015-a-biosimilar-infliximab-in-patients-with-active-rheumatoid-arthritis-on-stable-methotrexate-doses-54-week-results-of-a-randomized-double-blind-active-comparator-study/