Session Information
Date: Monday, November 13, 2023
Title: (1345–1364) Reproductive Issues in Rheumatic Disorders Poster II
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: We evaluated the role of maternal 25(OH)-vitamin D in adverse pregnancy outcomes in systemic lupus erythematosus (SLE).
Methods: We used a longitudinal cohort that included visits of pregnant patients with assessment of 25(OH)-vitamin D at each visit. Pregnancies were excluded if there was no vitamin D level during pregnancy, if the outcomes of pregnancies were missing (such as data on due date, birth date, gestational age, and complications of pregnancies), or if the pregnancy was terminated. 270 pregnancies were available for the statistical analysis.
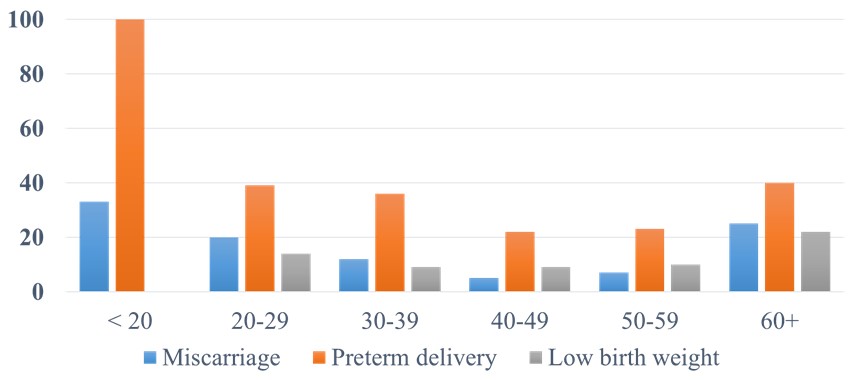
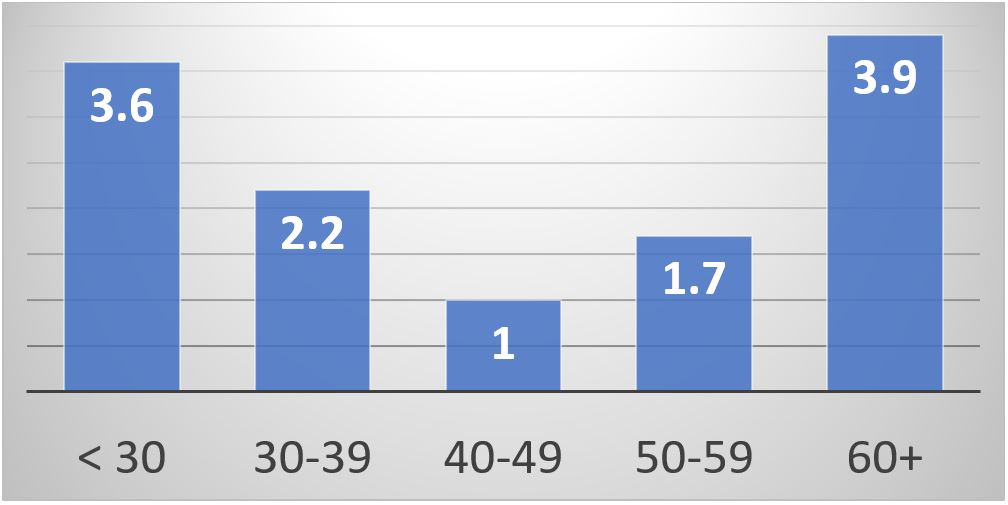
Results: We found a U-shape association between the maternal serum 25(OH)-vitamin D level and the combined adverse pregnancy outcomes (P=0.0022), miscarriage (P=0.0048), and preterm delivery (P=0.0003). We also found the same U-shape curve with the low birth weight; but it did not reach statistical significance due to the smaller number of cases. The lowest prevalence of adverse pregnancy outcomes was with 25(OH)-vitamin D in the range of 40-59 ng/dL (Figure 1). The multivariate analysis confirmed the same U-shape association controlling for age, race, and antiphospholipid antibodies (Table 1, Figure 2). The results were similar when we examined the subset in which pre-pregnancy or first trimester BMI were available.
Conclusion: Our study design cannot prove a cause-and-effect relationship. Most of our patients were prescribed vitamin D supplementation (but obviously many did not take it). We recommend monitoring 25(OH)-vitamin D levels during SLE pregnancies. The ideal 25(OH)-vitamin D range was 40-59 ng/dL.
To cite this abstract in AMA style:
Madanchi N, Fava A, Goldman D, Magder L, Jacobson R, Petri M. Both Low and High 25(OH)-Vitamin D Levels Increase Adverse Pregnancy Outcomes in Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/both-low-and-high-25oh-vitamin-d-levels-increase-adverse-pregnancy-outcomes-in-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/both-low-and-high-25oh-vitamin-d-levels-increase-adverse-pregnancy-outcomes-in-systemic-lupus-erythematosus/