Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: SLE is a chronic inflammatory disease triggered by the deposition of autoantibodies. One of the goals for SLE therapy is to suppress the production of the pathogenic antibodies. Bortezomib (Bz) is a proteasome inhibitor and directly targeting antibody-producing plasma cells. Recently, we reported the first randomized control trial to evaluate the effect of Bz for SLE (Ishii et al.. ACR 2015). In that study, we showed that Bz treatment is associated with many adverse reactions for refractory lupus patients. Previously, several reports showed that Bz was effective on and improved survival rate of lupus model mice. However, since Bz treatments for lupus model mice were initiated around the onset of diseases in these reports, it is not clear whether Bz has therapeutic effect on mice with high disease activity as well as preventive effect on low disease activity. In this study, we examined therapeutic effect of Bz on 14 wks-old (high disease activity) MRL/lpr mice and compared the survival curves of 10 wks-(around disease onset) and 14wks-Bz initiated mice.
Methods:
(1) Female MRL/lpr mice at 10 wks- and 14 wks- (n=8, each) were analyzed for anti-dsDNA antibody, weight of lymph nodes and spleen, and cellular subsets of spleen and bone marrow. (2) MRL/lpr mice (14 wks) were treated with (i) PBS (n=13), (ii) Bz (750ug/kg, twice in a week) (n=12), (iii) cyclophosphamide (Cyc) (1mg/body, once in 2 weeks) (n=15) and analyzed for anti-dsDNA antibody, pathological index of glomerulonephritis and plasma cell number at 24 wks-old. (3) Survival curve of 10 wks- and 14 wks-Bz initiated groups were compared.
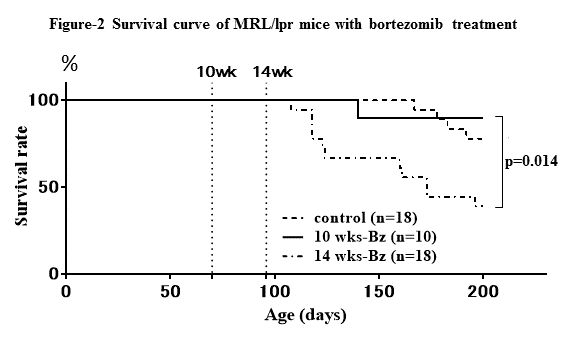
Results: MRL/lpr mice at 14 wks-old showed more weight of lymph nodes and spleen (p=0.0015, p=0.0013, respectively), and significantly higher anti-dsDNA antibody titer than those at 10 wks (p=0.033) (Fig-1), indicating higher disease activity at 14 wks old. Both treatments with Bz and Cyc significantly decreased the number of spleen cells (p<0.001, p=0.0136, respectively), glomerulonephritis index (p=0.0017, p=0.0034, respectively). Bz significantly decreased plasma cells (p<0.001) and anti-dsDNA antibody titer (p<0.001), while Cyc did not. In survival curve, 14 wks-Bz initiated group showed significantly higher mortality rate than control and 10 wks group (p=0.014) (Fig-2).
Conclusion: In spite of a therapeutic trial, Bz treatment had more toxic effect on mice with higher disease activity. Understanding the mechanism of the toxicity is important for clinical application of Bz to human SLE. 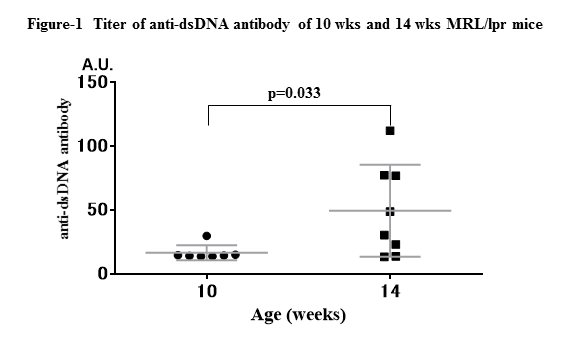
To cite this abstract in AMA style:
Fujii H, Ikeda T, Nose M, Muto T, Akita K, Kamogawa Y, Shirai T, Shirota Y, Ishii T, Harigae H. Bortezomib Treatment Induces Higher Mortality Rate in Lupus Model Mice with High Disease Activity [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/bortezomib-treatment-induces-higher-mortality-rate-in-lupus-model-mice-with-high-disease-activity/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bortezomib-treatment-induces-higher-mortality-rate-in-lupus-model-mice-with-high-disease-activity/
