Session Information
Session Type: Late-Breaking Abstracts
Background/Purpose:
To evaluate the efficacy, safety, and tolerability of subcutaneously-administered blisibimod (A-623, AMG 623), an inhibitor of soluble- and membrane-bound B-cell activating factor (BAFF), in patients with systemic lupus erythematosus (SLE) in the Phase 2b clinical trial, PEARL-SC.
Methods:
547 SLE patients with anti-double-stranded DNA or anti-nuclear antibodies and SELENA-SLEDAI score ≥6 at baseline were randomized 1:1 to receive placebo or blisibimod administered at 1 of 3 dose levels, 100 mg weekly (QW), 200 mg QW, or 200 mg every 4 weeks. Randomization was stratified by baseline SELENA-SLEDAI score (6-9 vs ≥10) and race. The primary endpoint was the percentage of subjects who achieved an SLE Responder Index-5 (SRI-5) response at Week 24 in the pooled blisibimod arms compared to the pooled placebo arms. An SRI-5 responder has: ≥5 point improvement in SELENA-SLEDAI, AND no new BILAG A or ≥2B organ domain scores, AND no worsening (<0.3 increase) in Physician's Global Assessment.
Results:
The primary endpoint of this study was not met primarily due to the lack of efficacy in the two lowest dose groups. However, SRI-5 responder rates were higher in subjects receiving blisibimod 200mg QW compared with pooled placebo, from Week 16 (DSRI‑5 for blisibimod-placebo=8%, p= 0.14), through Week 24 (DSRI‑5=8.2%, p=0.15), reaching statistical significance at Week 20 (DSRI‑5=13.4%, p = 0.02).
Treatment benefit was greater still when compared with the regimen-matched (QW) placebo. In pre-specified secondary analyses, benefit was observed at Week 24 with the 200mg QW group compared with matched placebo (n=92 and 92, respectively) using modified SRI analyses in which responders attained SELENA-SLEDAI improvements of ≥7 or ≥8 (DSRI‑5 = 8.7% p=0.23; DSRI-7 =16.3% p=0.003; DSRI-8 =17.4% p=0.001).
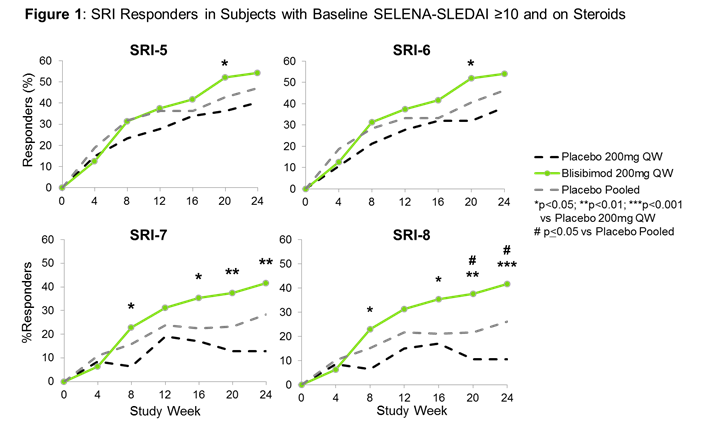
Blisibimod was also effective in a subgroup of SLE subjects with baseline SELENA-SLEDAI ≥10 and receiving corticosteroids at any dose (n=278) utilizing more stringent response thresholds (DSRI-5=13.8%, p=0.18; DSRI-7=28.9%, p=0.002; DSRI-8=31.1%, p<0.001, Figure 1). More than 82% of the subjects in this subgroup had mucocutaneous or musculoskeletal disease.
As expected with its mechanism of BAFF inhibition, blisibimod treatment resulted in significant reductions in total B cells and anti-dsDNA antibodies, and significant increases in C3 and C4.
Blisibimod was safe and well-tolerated at all dose levels with no meaningful imbalances in serious adverse events or infections compared with placebo. Amongst the commonly-reported AEs, only injection site reactions occurred more frequently with blisibimod compared with placebo (15% and7%, respectively), but were never serious or severe.
Conclusion:
These data support further evaluation of 200mg QW blisibimod using more stringent thresholds of the SRI in patients with SLE, including mucocutaneous or musculoskeletal disease.
Disclosure:
R. A. Furie,
Anthera Pharmaceuticals Inc,
5;
M. A. Scheinberg,
None;
G. Leon,
None;
E. B. Ramiterre,
None;
M. Thomas,
None;
R. S. Martin,
Anthera Pharmaceuticals Inc,
1,
Anthera Pharmaceuticals Inc,
3;
M. Petri,
Anthera Pharmaceuticals Inc,
2,
Anthera Pharmaceuticals Inc,
5.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/blisibimod-an-inhibitor-of-b-cell-activating-factor-in-patients-with-moderate-to-severe-systemic-lupus-erythematosus/