Session Information
Date: Tuesday, November 7, 2017
Title: Pediatric Rheumatology – Clinical and Therapeutic Aspects Poster III: Juvenile Arthritis
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Despite their increasing use in adults, there is little to no data available on the use of biosimilar drugs in children with JIA, despite anecdotal evidence of their use in clinical practice. This analysis aims to describe the characteristics of children and young people (CYP) with JIA starting biosimilars in the UK over the first 2 years following their approval in the United Kingdom (UK) for adults with musculoskeletal diseases.
Methods: The Biologics for Children with Rheumatic diseases (BCRD) study, launched in 2010, is an ongoing prospective UK study of children with JIA starting biologic therapies other than etanercept (followed in a separate parallel study). Baseline information is collected via questionnaires completed by the treating physician or affiliated clinical research nurse. Follow-up data including disease activity measures and changes in drug therapy are collected at 6 months, 1 year and annually thereafter. Since 30/09/2015, data has been captured on 3 biosimilars available in the UK: infliximab (Inflectra and Remsima) and etanercept (Benepali).
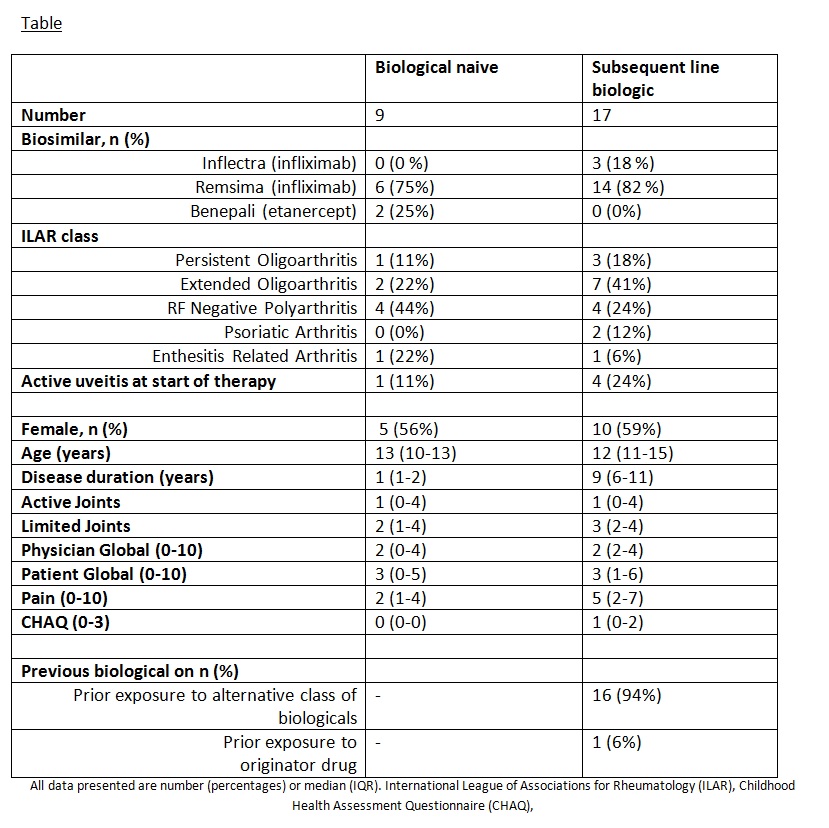
Results: To 10/05/2017, 26 patients were identified in the BCRD study starting a biosimilar: 21 (81%) Remsima, 3 (12%) Inflectra and 2 (8%) Benepali). Of these, 9 (35%) started a biosimilar as their first biologic therapy. Only 1 patient starting Remsima switched directly from the originator product, Remicade. Sixteen (62%) switched from an alternative non-originator biologic (table). Reasons for switching from these alternative biologics were efficacy reasons (n=8, 50%), safety reason (n=5, 31%), efficacy and safety issues combined (n=2, 13%) and needle phobia (n=1, 6%). Six-month and 1 year follow-up data were available in 3 and 1 CYP respectively. No serious adverse events have been reported to date and all 4 CYP continue on their biosimilar drug.
Conclusion: This preliminary study gives a first overview of initial biosimilar use in CYP with JIA in the UK. It has shown that these drugs are used as both first-line and subsequent-line biologic therapy despite a lack of license for this indication. Unlike evidence from Rheumatoid Arthritis, where a majority of patients receiving biosimilars to date have switched from the originator, this initial experience in JIA suggests that biosimilars are being considered as front line therapeutic option instead of the originator, presumably as a cost-saving measure. Further follow-up of these children will assess the effectiveness and safety of these products in paediatric use.
To cite this abstract in AMA style:
De Cock D, Kearsley-Fleet L, Baildam E, Beresford MW, Foster HE, Southwood TR, Thomson W, Hyrich KL. Biosimilar Use in Children and Young People with Juvenile Idiopathic Arthritis in a Real-World Setting in the United Kingdom [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/biosimilar-use-in-children-and-young-people-with-juvenile-idiopathic-arthritis-in-a-real-world-setting-in-the-united-kingdom/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/biosimilar-use-in-children-and-young-people-with-juvenile-idiopathic-arthritis-in-a-real-world-setting-in-the-united-kingdom/