Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Clinical equivalence has been shown for BI 695501 and the adalimumab reference product (RP) through similar ACR20 response at Weeks (wks) 12 and 24 in patients (pts) with moderately-to-severely active RA in the Phase III VOLTAIRE®-RA study (Cohen et al 2017. EULAR 2017. Abst FRI0189). Here we present the long-term safety, efficacy and immunogenicity data.
Methods: In this 58-wk, double-blind, multicenter, Phase III active-comparator study (NCT02137226), 645 adalimumab-naive adults with moderately-to-severely active RA receiving stable treatment with methotrexate (prior exposure to one biologic allowed) were randomized (1:1) to BI 695501 or adalimumab RP 40 mg and treated every 2 wks up to Wk 24. At Wk 24, RP pts were re-randomized to switch to BI 695501 (SWITCH; n = 147 (full analysis set) / 146 (safety analysis set [SAF])), or to continue RP (RP-CONT; n = 148) until Wk 48; BI 695501 pts were dummy re-randomized and continued BI 695501 (BI 695501-CONT; n = 298). Data from pts treated only with BI 695501 (N=324) or RP (N=175) for the entire 48 wks were also analysed. Efficacy data were collected until Wk 48. Safety follow-up was to Wk 58 for all pts who did not enter the open-label extension trial (VOLTAIRE®-RAext).
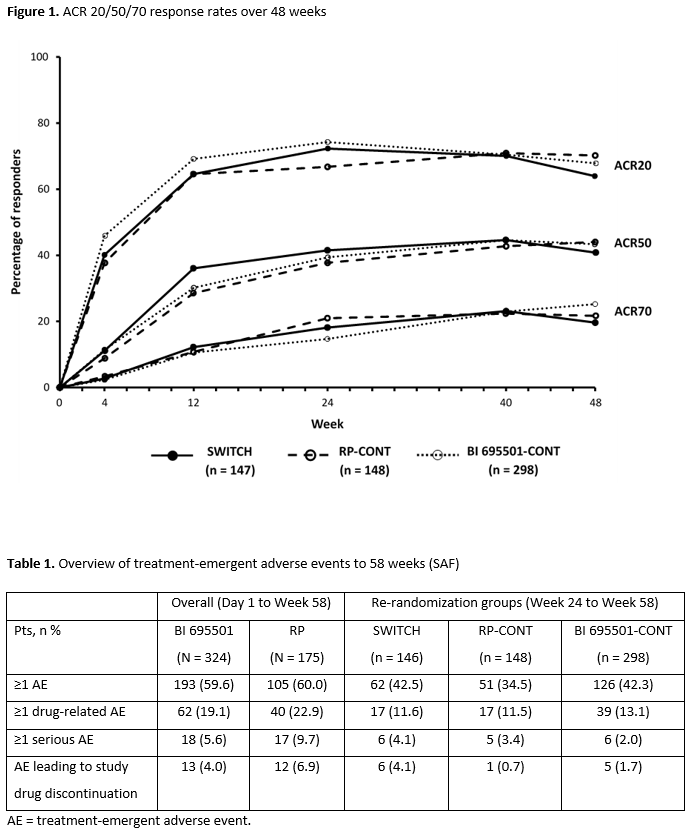
Results: Baseline demographics were balanced across treatment groups. ACR20/50/70 response rates were similar across the groups up to Wk 48 (Figure 1). At Wk 48, mean changes from baseline in DAS28-ESR were –2.71, –2.60, and –2.70 in the SWITCH, RP-CONT, and BI 695501-CONT groups, respectively. Safety findings were similar between continuous arms from Day 1 to Wk 58, and re-randomization groups from Wk 24 to Wk 58 (Table 1). Among serious adverse events (SAEs), infections and infestations was the most common system organ class (0.6% for BI 695501 vs 4.0% for RP). No deaths were reported during the study. Similar immunogenicity (anti-drug antibody [ADA] frequency and titers, neutralizing antibody [nAb] frequency) was detected up to Wk 48 in all re-randomized groups. The proportion of pts with positive ADA response at Wks 24 and 48 was 44.5% and 36.2% for the SWITCH group, 50.3% and 49.6% for RP-CONT, and 42.8% and 41.8% for BI 695501-CONT. Positive nAb response frequency at Wk 24 and 48 was 15.8% and 15.2% (SWITCH), 23.8% and 21.6% (RP-CONT), and 15.8% and 19.1% (BI 695501-CONT). The single transition from RP to BI 695501 had no impact on efficacy, safety, and immunogenicity.
Conclusion: These data confirm that BI 695501 and adalimumab RP have similar efficacy, safety, and immunogenicity in pts with RA over 48 wks of treatment. These findings also held true for pts switching from adalimumab RP to BI 695501 at Wk 24.
Acknowledgments:
The authors would like to thank Deepak Assudani, Ivo Sonderegger, Michael Hug, Benjamin Lang, Susanne Buschke and Christina Grundt, who each provided substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work.
To cite this abstract in AMA style:
Cohen SB, Alonso-Ruiz A, Klimiuk PA, Lee E, Peter N, Czeloth N, Jayadeva G. Biosimilar Candidate BI 695501 and Adalimumab Reference Product Have Similar Efficacy and Safety in Patients with Moderately-to-Severely Active Rheumatoid Arthritis (RA): 1-Year Results from a Phase III Study [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/biosimilar-candidate-bi-695501-and-adalimumab-reference-product-have-similar-efficacy-and-safety-in-patients-with-moderately-to-severely-active-rheumatoid-arthritis-ra-1-year-results-from-a-phase-i/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/biosimilar-candidate-bi-695501-and-adalimumab-reference-product-have-similar-efficacy-and-safety-in-patients-with-moderately-to-severely-active-rheumatoid-arthritis-ra-1-year-results-from-a-phase-i/