Session Information
Session Type: Poster Session B
Session Time: 10:30AM-12:30PM
Background/Purpose: In 2023 patent exclusivity for adalimumab expired and nine biosimilars were introduced to the U.S. market with a tenth launched in May 2024. The arrival of these biosimilars was eagerly awaited given the potential to reduce costs and improve access however uptake in the first year of availability was very low. CVS Caremark removed adalimumab from its national formulary in favor of adalimumab-adaz in April 2024. Here we assess how this decision changed the uptake of -adaz and other biosimilar adalimumab molecules in community rheumatology practices.
Methods: Data derive from RISE, a national EHR database from over 1100 US rheumatologists. We included patients age > 18 years, with Medicare, Medicaid or private insurance, prescribed adalimumab (reference or biosimilar) in 2023 or the first 9 months of 2024 (due to data availability). Covariates include age, sex, race-ethnicity, and area deprivation index. Psoriatic arthritis (PSA), ankylosing spondylitis (AS), or rheumatoid arthritis (RA) was defined by two ICD codes at least 30 days apart with patients assigned to one based on the clinically adjudicated hierarchy: PSA >AS >RA >other. We tested for differences in ever use of a biosimilar between years as well as ever use in 2023/2024 combined across insurance coverage, demographics, and diagnosis using standardized mean differences. We used an interrupted time series (ITS) to model the effect of the CVS formulary change on the proportion of prescriptions (per month) for -adaz and all other biosimilars combined. Our final model is an ITS with interruptions at February 2024 and May 2024 to best characterize the observed pattern for -adaz, and a linear regression to characterize the other biosimilars (as ITS estimates suggested no discontinuity).
Results: 22,079 patients received adalimumab or a biosimilar during our study period. In 2023 < 4% of patients received a biosimilar whereas in the first 9 months of 2024 this jumps to nearly 25% of patients (Table 1). 16.4% of patients with private insurance, 8.2% on Medicaid, and 6.5% with Medicare were ever on a biosimilar. There were only trivial differences by sex, race-ethnicity, ADI and diagnosis (Table 1). In 2024 most biosimilar prescriptions were -adaz (55.5%) followed by -bwwd (19%) (Table 2). There were nearly no prescriptions for -adaz Jan 2023–Jan 2024 but a >13% jump in prescriptions in Feb 2024. Uptake continued to rise at the rate of nearly 3% per month through May 2024. There was a 9.3% drop from April to May with use continuing to decline thereafter at a rate of 0.7% per month (Figure 1). Prescriptions for the other biosimilars increased steadily at a rate of 0.7% per month over the study period.
Conclusion: The CVS formulary change had a dramatic effect on prescriptions. The increase started 2 months before the official date, which likely reflects proactive changes in ordering practices by clinicians and pharmacists. The large decrease immediately after the formulary change has not been reported elsewhere. This may reflect the nocebo effect, patient preference for certain reference product characteristics (e.g., citrate free formulation), or switching to a different product after the removal of manufacturer copay assistance programs.
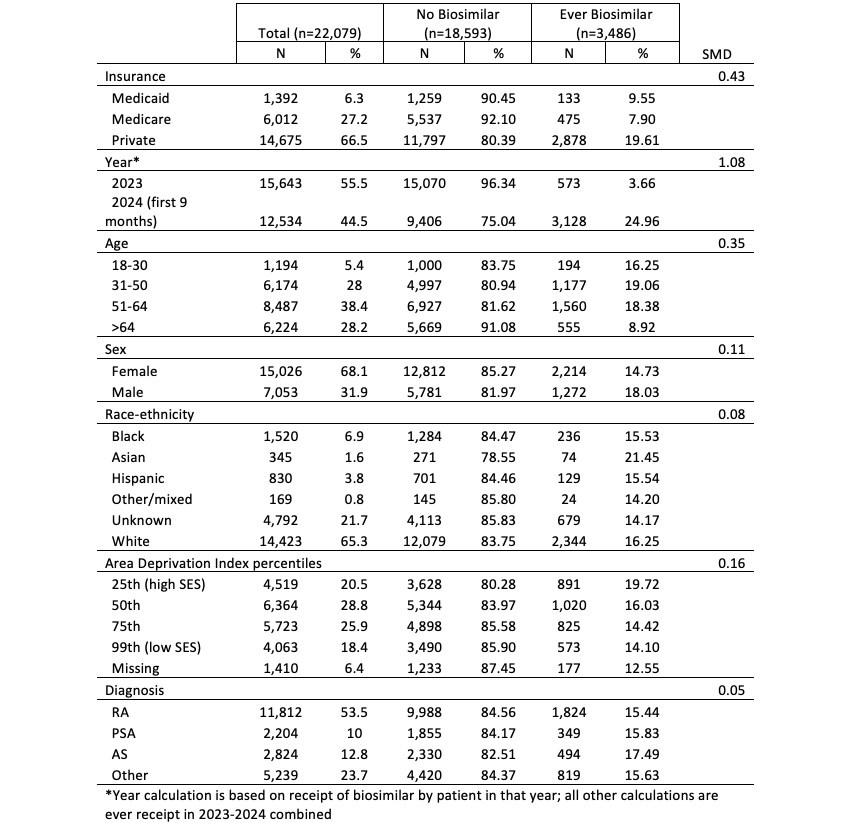 Table 1. Demographic and clinical characteristics of patients prescribed adalimumab or its biosimilars in RISE 2023-2024.
Table 1. Demographic and clinical characteristics of patients prescribed adalimumab or its biosimilars in RISE 2023-2024.
.jpg) Table 2. Count of distinct patients prescribed adalimumab or its biosimilars in RISE by year.
Table 2. Count of distinct patients prescribed adalimumab or its biosimilars in RISE by year.
.jpg) Figure 1. Interrupted time series analysis of the proportion of prescriptions dispensed that were adalimumab-adaz and linear regression of other adalimumab biosimilars by month. Each dot represents the actual proportion of prescriptions dispensed. Disclaimer: This data was supported by the ACR’s RISE Registry. However, the views expressed represent those of the authors, not necessarily those of the ACR.
Figure 1. Interrupted time series analysis of the proportion of prescriptions dispensed that were adalimumab-adaz and linear regression of other adalimumab biosimilars by month. Each dot represents the actual proportion of prescriptions dispensed. Disclaimer: This data was supported by the ACR’s RISE Registry. However, the views expressed represent those of the authors, not necessarily those of the ACR.
To cite this abstract in AMA style:
Roberts E, Schmajuk g, Ung D, Clark M, Yazdany J. Biosimilar adalimumab prescriptions increase before the CVS formulary change and decrease after [abstract]. Arthritis Rheumatol. 2025; 77 (suppl 9). https://acrabstracts.org/abstract/biosimilar-adalimumab-prescriptions-increase-before-the-cvs-formulary-change-and-decrease-after/. Accessed .« Back to ACR Convergence 2025
ACR Meeting Abstracts - https://acrabstracts.org/abstract/biosimilar-adalimumab-prescriptions-increase-before-the-cvs-formulary-change-and-decrease-after/
