Session Information
Date: Sunday, November 7, 2021
Title: Spondyloarthritis Including PsA – Treatment Poster I: Axial Spondyloarthritis (0908–0939)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
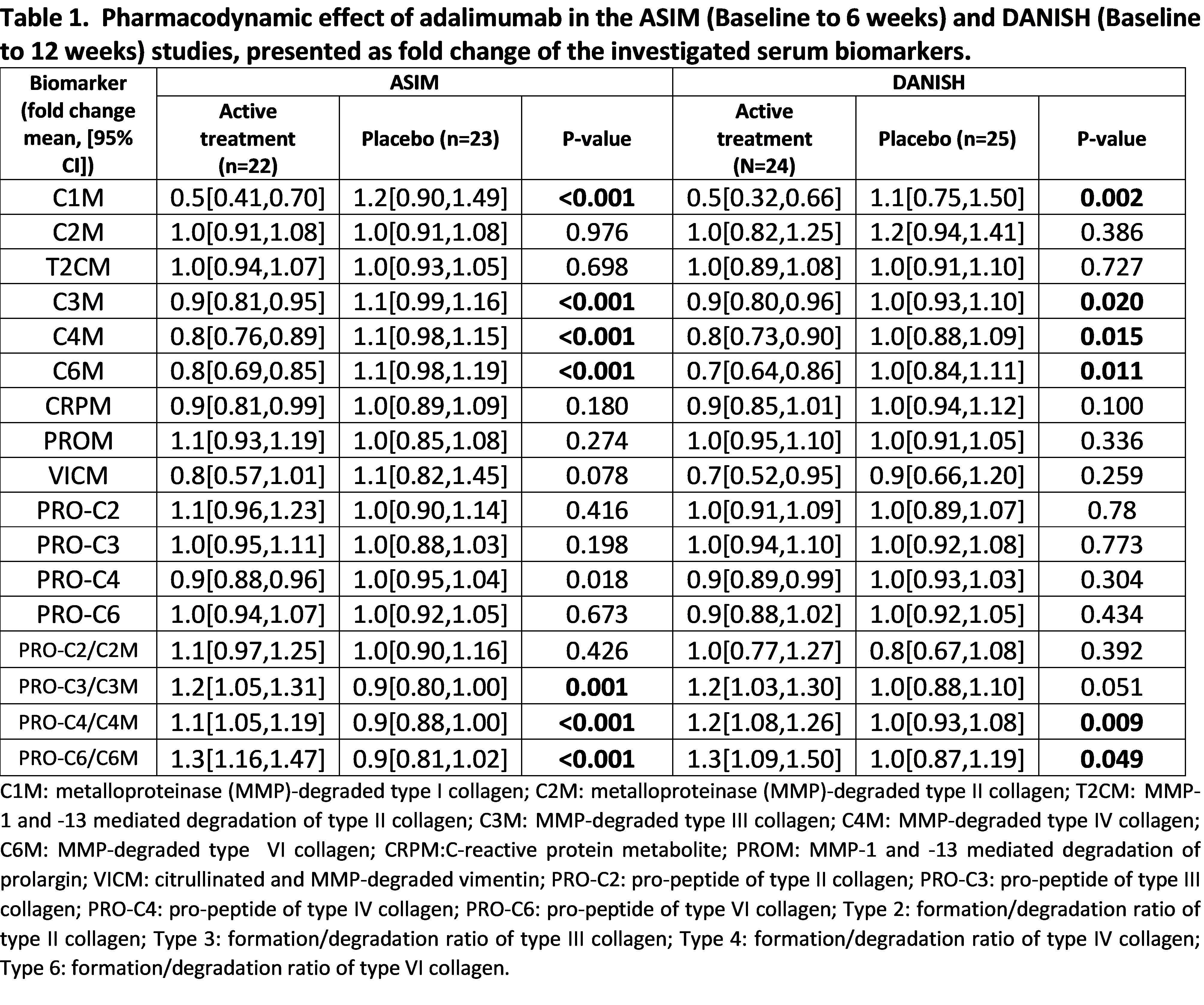
Background/Purpose: Axial spondyloarthritis (axSpA) is a chronic inflammatory disease associated with extracellular matrix (ECM) remodeling of the cartilage, bone, and connective tissues. Quantification of ECM-mediated biomarkers may be useful to determine treatment response in axSpA. The aim of this study was to identify biomarkers reflecting the pharmacodynamic effect and the treatment response of adalimumab in two placebo-controlled axSpA studies.
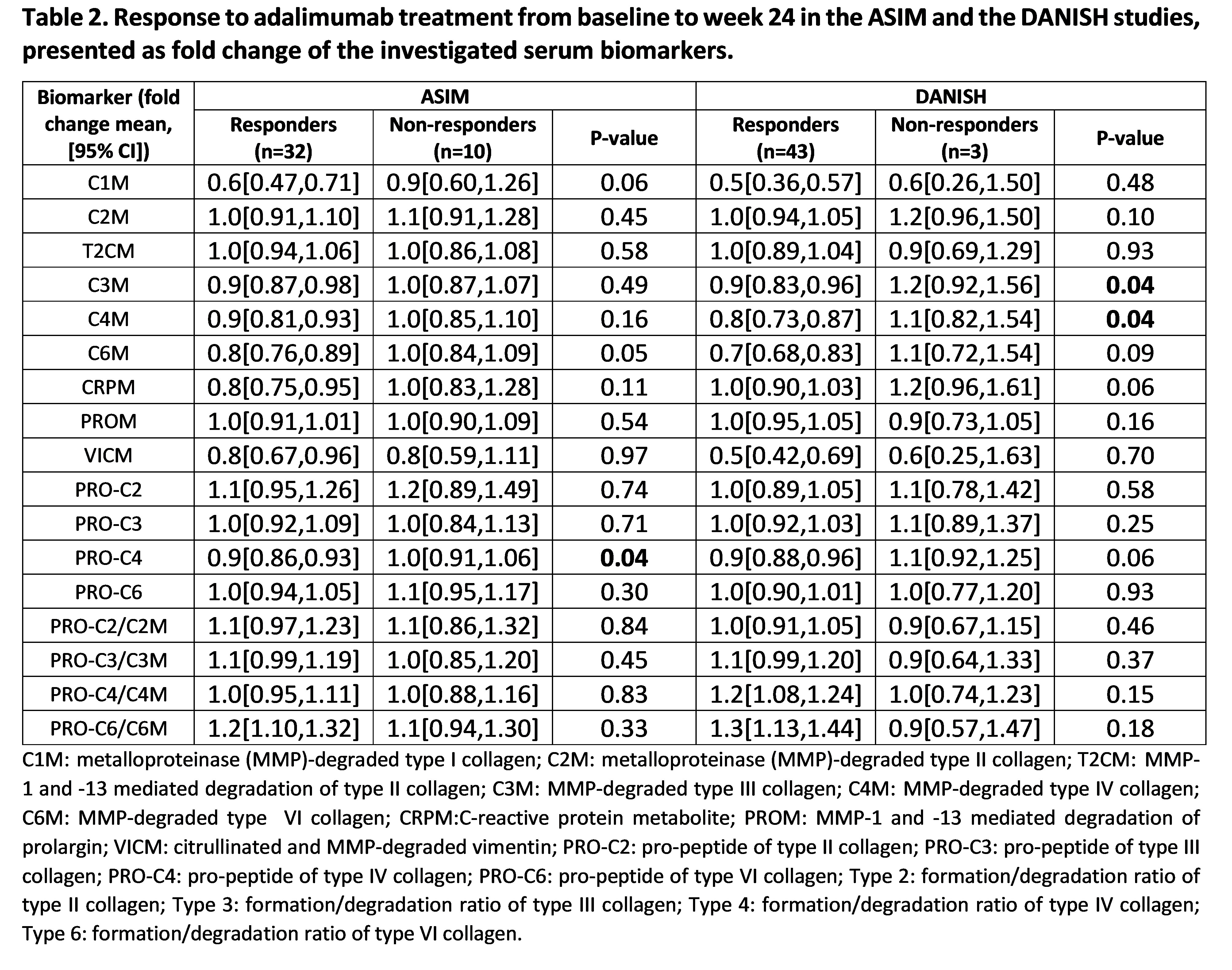
Methods: Biomarkers reflecting the degradation of collagen type I (C1M), II (C2M and T2CM), III (C3M), IV (C4M), VI (C6M), formation of collagen type II (PRO-C2), III (PRO-C3), IV (PRO-C4) and VI (PRO-C6) and inflammation (CRPM, PROM, and VICM) were measured in serum from the ASIM study (n=45) and the DANISH study (n=49) at baseline, week 6 or 12 (ASIM and DANISH, respectively), and week 24. Patients received treatment with adalimumab 40 mg or placebo the first 6 or 12 weeks (ASIM and DANISH, respectively) followed by adalimumab 40 mg for an additional 18 or 12 weeks (ASIM and DANISH, respectively). Differences in fold change of biomarker levels between week 6 or week 12 (ASIM and DANISH, respectively) and baseline were evaluated by a linear regression model to investigate treatment pharmacodynamics. A linear regression model adjusted by treatment (active treatment or placebo for the first 6 or 12 weeks, ASIM and DANISH, respectively) were applied to investigate treatment response. Response to treatment was evaluated as a reduction of at least 50% in BASDAI index from baseline to week 24.
Results: In the ASIM vs. DANISH study 56% vs. 78% of the patients were male, the mean (SD) age of the patients was 37.6 (9.9) vs. 39.5 (11.2) and frequency of HLA-B27 78% vs. 88%. The 22 patients representing the primarily actively treated group in the ASIM study presented significantly lower fold change in C1M, C3M, C4M, C6M, and PRO-C4 over the first 6 weeks as compared to the placebo group (p< 0.001, p< 0.001, p< 0.001, p< 0.001, p= 0.018, respectively Table 1), and showed a significantly higher fold change in the ratios of formation/degradation of collagen type III, IV and VI (all p< 0.001). The 24 patients from the actively treated group in the DANISH study had a significantly lower fold change over the first 12 weeks in C1M, C3M, C4M, C6M as compared to the placebo group (p=0.002, p=0.02, p=0.015, p=0.011, respectively, Table 1), and had a significantly higher fold change in the ratios of formation/degradation of collagen type IV and VI (p=0.009, p=0.049, respectively). Thirty-two out of 45 patients were considered responders in the ASIM study, and 43 out of 46 patients in the DANISH study (Table 2). In the ASIM study, levels of PRO-C4 presented a significantly lower fold change in responders compared to non-responders (p=0.042), while in the DANISH study, levels of C3M and C4M had both a significantly lower fold change in responders compared to non-responders (both p=0.04, Table 2).
Conclusion: The biomarkers C1M, C3M, C4M, and C6M may have potential as pharmacodynamic biomarkers, while the biomarkers C3M and C4M were associated with response to adalimumab treatment after 24 weeks.
To cite this abstract in AMA style:
Port Linares H, Nielsen S, Frederiksen P, Falkenløve Madsen S, Bay-Jensen A, Karsdal M, Sørensen I, Jensen B, Gitte Loft A, Rintek Madsen O, Ostergaard M, Pedersen S. Biomarkers of Extracellular Matrix Turnover Reflect Treatment Response and Pharmacodynamic Effects of TNF-α Inhibitory Therapy in Patients with Axial Spondyloarthritis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/biomarkers-of-extracellular-matrix-turnover-reflect-treatment-response-and-pharmacodynamic-effects-of-tnf-%ce%b1-inhibitory-therapy-in-patients-with-axial-spondyloarthritis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/biomarkers-of-extracellular-matrix-turnover-reflect-treatment-response-and-pharmacodynamic-effects-of-tnf-%ce%b1-inhibitory-therapy-in-patients-with-axial-spondyloarthritis/