Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The FDA approved the first biologic for the treatment of nr-axSpA in the US in March 2019. The objective of our study was to describe biologic use and reasons for switching biologic therapy among patients with non-radiographic axial spondyloarthritis (nr-axSpA).
Methods: Data from a real world, cross-sectional survey of rheumatologists and their consulting nr-axSpA patients in the United States were analyzed descriptively. Data were collected from Jun-Aug 2018 via physician-completed patient record forms and patient self-completed forms. Patients who had physician confirmed diagnosis of nr-axSpA were eligible to participate. Rheumatologists completed record forms for their patients, containing information on current medication use and reasons for switching biologics.
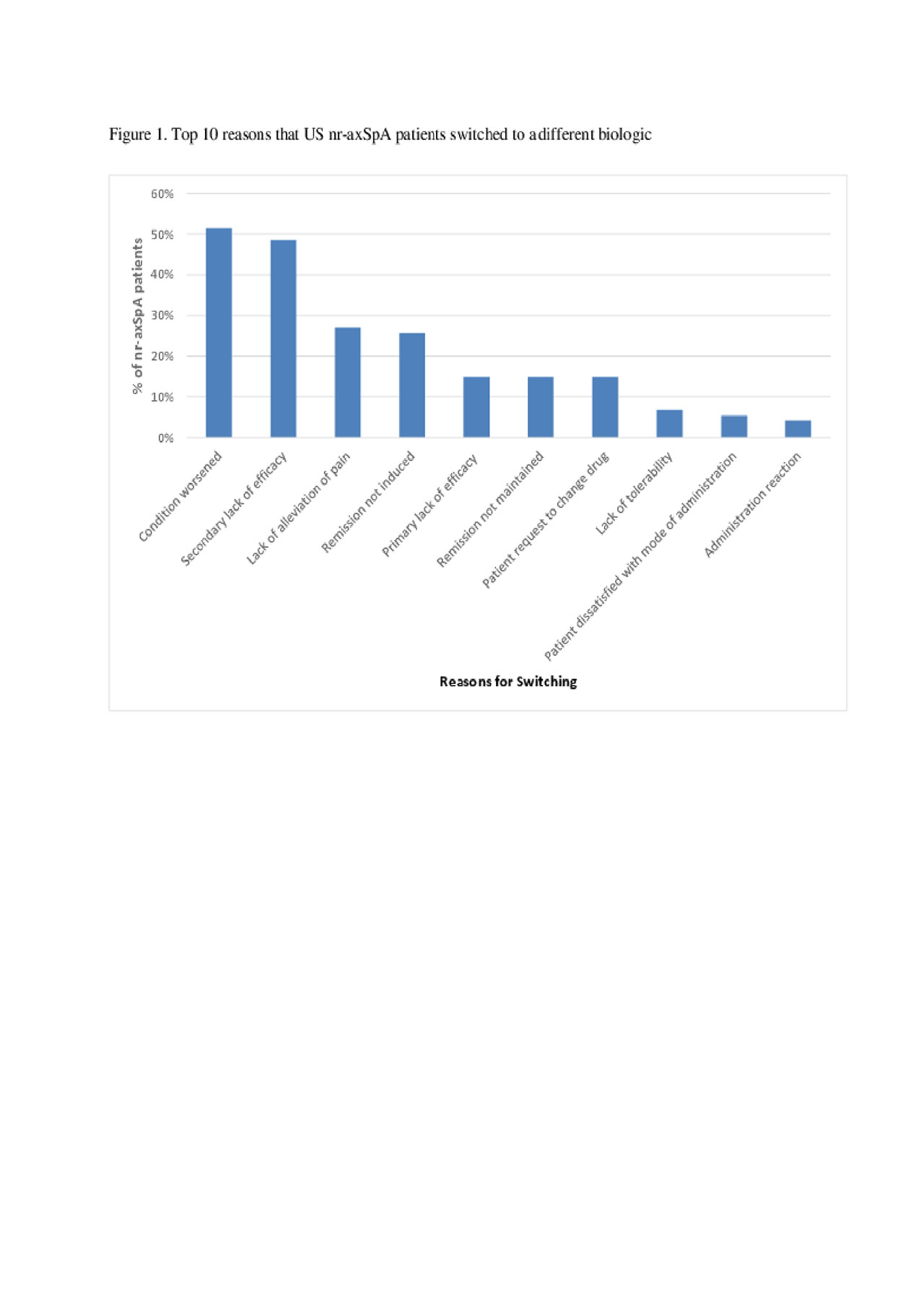
Results: Data from 88 rheumatologists and 495 nr-axSpA patients were included in this analysis. The majority of nr-axSpA patients were male (53.3%), with a mean age of 44.2 years, and 69.8% of patients reported working full-time. Of the 495 nr-axSpA patients, 48.1% were receiving a biologic agent and no csDMARD, 18.4% csDMARD (no biologic), 18.2% NSAIDS/COX-2 (no biologic or csDMARD), 11.5% a biologic and a csDMARD, 2.0% were receiving no therapy, and 1.8% other therapy (no biologic, csDMARD or NSAID/COX-2). Of the 295 patients receiving a biologic, 78.0% were receiving their first, 13.9% their second and 8.1% their third or more biologic. Of the 74 nr-axSpA patients that switched from a previous biologic to their current biologic, physicians reported that 51.4% switched due to condition worsening, 48.6% had a loss of response over time, 27.0% switched due to a lack of pain alleviation, and 25.7% of patients switched because remission was not induced (Figure 1).
Conclusion: This survey suggests that around 60% of nr-axSpA patients were receiving biologic therapy. Switching of biologics is frequent in nr-axSpA patients and is usually due to lack of efficacy, loss or response, and effort to accomplish remission.
To cite this abstract in AMA style:
Deodhar A, Hunter T, Holdsworth E, Booth N, Sandoval D. Biologic Use and Reasons for Switching Biologic Therapy in Patients with Non-radiographic Axial Spondyloarthritis in the United States:Findings from a US Survey [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/biologic-use-and-reasons-for-switching-biologic-therapy-in-patients-with-non-radiographic-axial-spondyloarthritis-in-the-united-statesfindings-from-a-us-survey/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/biologic-use-and-reasons-for-switching-biologic-therapy-in-patients-with-non-radiographic-axial-spondyloarthritis-in-the-united-statesfindings-from-a-us-survey/