Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Multiple biologic DMARDs (bDMARDs) are approved in the US for the treatment of RA. Previous studies have indicated significant clinical inertia in moving patients who have failed conventional DMARDs (cDMARDs) to bDMARDs. We sought to identify factors associated with bDMARD use.
Methods : The data for this analysis were obtained from the 2015 online RA community survey “Rheumatoid Arthritis in America”. The survey was administered by Health Union between August 6, 2015 and Sep. 12, 2015. Subjects self-identified as having RA and were recruited from RheumatoidArthritis.net subscribers, email and social media (N=3149). Patients aged 18 years or older at RA diagnosis, living in the US, and naïve to clinical trials (N=2735) were included in this analysis. Survey variables included demographics; financial status; disease onset and disease status; patient reported outcomes; RA therapy; provider/practice characteristics; patient knowledge, attitudes, perceptions of RA (disease, therapy, and care), patient behaviors; and medication history. A regression tree partitioning algorithm was used as the primary analysis to systematically identify subgroups of patients where bDMARD use was substantially different from the overall population. As a secondary analysis, logistic regression with stepwise selection was also performed.
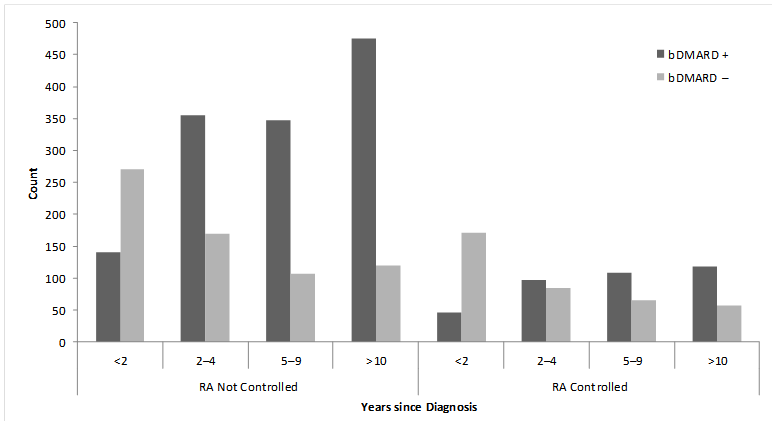
Results : Patients were mostly women (96%), Caucasian (87.9%), mean age was 51.5 years, and mean time since diagnosis of 7.3 years. Overall, 72.6% of patients had signs and symptoms that suggested their RA disease was not controlled and 38.2% were naïve to bDMARDs. Ever use of a bDMARD was higher in uncontrolled patients (vs. patients who had evidence of disease control); this observation was consistent across disease duration (Figure). Of 47 subgroup variables used in the regression tree algorithm, three variables were associated with bDMARD use and met the stringent selection criteria (consistency, internal/external validity): (1) time since RA diagnosis (≥2 years); (2) expected annual out of pocket spending (≥$2000); and (3) preferred medication route of administration (prefer injection/infusion). The stepwise logistic regression retained 18 variables including the three variables identified by regression tree analysis. Of the remaining 15 variables, odds ratios for ever use of bDMARDs greater than 2.0 were observed among patients who were uninsured, were treated by a rheumatologist, had moderate/severe disease at symptom onset or currently, and prior use of cDMARDs.
Conclusion : In this online community of RA patients, 72.6% did not have disease in control and 38.2% had never used a biologic. bDMARD use varied considerably among patient subgroups, particularly by time since RA diagnosis, expected annual out-of-pocket spending, and preferred medication route of administration.
Figure. Ever use of bDMARDs by disease status and years since diagnosis (n=2735).
To cite this abstract in AMA style:
Chang L, Tanaka Y, Larmore CJ, Qian L, Zhu B, Araujo AB. Biologic DMARD Use Among U.S. Patients in an Online Rheumatoid Arthritis Community [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/biologic-dmard-use-among-u-s-patients-in-an-online-rheumatoid-arthritis-community/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/biologic-dmard-use-among-u-s-patients-in-an-online-rheumatoid-arthritis-community/