Session Information
Date: Sunday, October 21, 2018
Title: Rheumatoid Arthritis – Treatments Poster I: Strategy and Epidemiology
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Biologic discontinuation in rheumatoid arthritis: a population based study 1999-2017.
Michael D Richter, Eric L Matteson, John M Davis III, Sara J Achenbach, Cynthia S Crowson
Background/Purpose: Discontinuation of biologic medications in rheumatoid arthritis (RA) can be used as a proxy for drug efficacy when direct measures of disease activity are not available. Recent data comparing the discontinuation rates of different biologics, specifically tumor necrosis factor (TNF) vs. non-TNF, have been mixed. Further, most of the existing data on this topic comes from controlled trials or cohorts of referred patients. Our objective was to determine drug discontinuation rates for TNF and non-TNF biologics and identify predictors and indications for discontinuation in a population based cohort.
Methods: We retrospectively studied 606 patients with incident RA from 1999-2013 in a geographically well-defined population. All patients met the 1987 American College of Rheumatology classification criteria for RA and were followed until July 1, 2016. Discontinuation rates were estimated using cumulative incidence adjusted for the competing risk of death. Cox models were used to examine potential predictors of discontinuation.
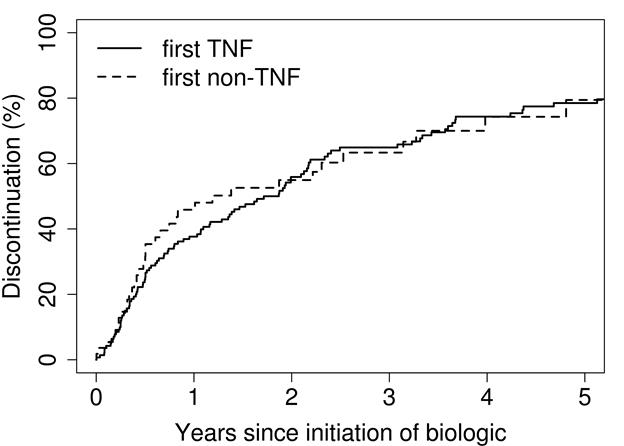
Results: 156 patients were treated with at least one biologic medication (mean age at first biologic: 52 years; 71% female; median years from RA diagnosis to first biologic: 1.3; median follow-up: 6.0 years). Cumulative incidence for time to discontinuation for first TNF biologic was 38% at 1 year (95% confidence interval [CI], 30-47%) and 56% at 2 years (95% CI, 48-65%). For first non-TNF biologic, these values were 46% at 1 year (95% CI, 34-62%) and 55% at 2 years (95% CI, 42-71%). The most common reasons for discontinuation of any first biologic were inefficacy (43%), adverse effects (15%), and achievement of therapeutic goal (13%). The frequencies of each reason were similar between TNF and non-TNF treated patients. Potential predictive factors for discontinuation, including age, sex, years from RA diagnosis, rheumatoid factor/anti-citrullinated protein antibody positivity, smoking, and obesity, did not reach statistical significance with the exception of age≥65 years, which was associated with increased risk for discontinuing non-TNF biologics (hazard ratio: 3.06; 95% confidence interval: 1.52-6.16).
Conclusion: Discontinuation rates were similar between TNF and non-TNF biologics. We did not identify any significant predictors of drug discontinuation apart from age in this population.
To cite this abstract in AMA style:
Richter M, Matteson EL, Davis JM III, Achenbach SJ, Crowson CS. Biologic Discontinuation in Rheumatoid Arthritis: A Population Based Study 1999-2017 [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/biologic-discontinuation-in-rheumatoid-arthritis-a-population-based-study-1999-2017/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/biologic-discontinuation-in-rheumatoid-arthritis-a-population-based-study-1999-2017/